Valsartan Lawsuit Overview
Valsartan is a prescription drug approved by the U.S. Food and Drug Administration (FDA) in 1996 to treat high blood pressure, hypertension, and heart conditions. It was initially marketed by Novartis under the brand name Diovan.
It is believed that hundreds of batches of the prescription drug were contaminated with NDMA, a cancer-causing agent. The discovery of this contamination led to global recalls, including by the FDA and European health officials. NDMA issues found in batches of the drug are believed to be similar to those in Zantac lawsuits.
Recalls of valsartan, which is available under multiple names, began in 2018, especially with the generic form of the drug. The widespread use of valsartan-containing drugs has led to a significant number of lawsuits. It is believed that millions may be affected. To date over 1200 lawsuits have been consolidated in a Multidistrict Litigation (MDL) in New Jersey. Affected individuals are strongly encouraged to seek legal representation.
Valsartan Lawsuit Updates – April 2024
April 9, 2024: No New Trial Date Scheduled for Valsartan Lawsuits
There is still no new trial date scheduled in the Valsartan Lawsuit. There have been several pre-trial motions decided in this bellwether trial. The federal court filing system known as “PACER” shows that a status conference occurred on March 27, 2024, and the transcript was posted under seal the next day. Whatever happened at that conference seems important. We think it is unlikely that the Valsartan trial is going forward this month since there is no trial date officially set at this point.
April 1, 2024: Valsartan Class Action Certification and Personal Injury Claims
The valsartan lawsuit has two distinct parts. First, there is the class action lawsuit. The Valsartan class action was certified by the US District Court for New Jersey in December of 2023. The class action certification does not decide whether any defendant did anything wrong; it simply allows different classes of plaintiffs to bring their cases in an efficient manner. The classes in the Valsartan class action lawsuit are: 1) consumer economic loss class, 2) medical monitoring class, and 3) the Third-Party Payor Class. In addition to the class action lawsuit, there are personal injury claims, which are separate from the class action. The personal injury cases allege that contaminated generic Valsartan caused cancer. Our firm is investigating valsartan cancer cases.
March 26, 2024: Key Decision Issued Ahead of Valsartan Trial by Judge Kugler
Judge Kugler has issued an important decision in advance of the Valsartan bellwether trial that was supposed to start 10 days ago. The Order, issued today, largely denies the request for decisions as a matter of law. Both plaintiffs and the defendants asked Judge Kugler to rule on at least 16 issues based on the documents and deposition testimony that have been produced so far. The Judge did, however, grant one important request made by the Plaintiffs. He ruled that defendants’ affirmations, statements, and labeling of the generic Valsartan they produce was an “express warranty.” Since the judge has already ruled that the statements of the defense were express warranties, they will not be able to challenge whether or not there were express warranties in front of the Jury. There is not yet a new trial date in the Valsartan MDL. We expect a date soon.
March 21, 2024: Study Links NDMA-Contaminated Valsartan to Increased Cancer Risk
Several scientific studies have linked the use of Valsartan contaminated with NDMA to an increased risk of cancer. One study compared 1.4 million people and their cancer rates. The control group consisted of the population that was taking Valsartan before the contamination date, and the other group took the contaminated Valsartan. The study was titled “Nitrosodimethylamine-Contaminated Valsartan and Risk of Cancer: A Nationwide Study of 1.4 Million Valsartan Users.” It was published in the Journal of the American Heart Association on December 20, 2022.
March 20, 2024: Valsartan Lawsuit Status Update
The Valsartan trail has been postponed. There is some indication that a member of the Court has become ill. There are also several pretrial motions that have not been decided yet. It is unclear when the trial will begin. It is possible that the court could grant summary judgment and say that certain manufacturers provided contaminated drugs. Plaintiff’s lawyers argue that Valsartan was clearly contaminated and several government findings confirms that much of the Valsartan sold between 2015 and 2018 was not pure. If summary judgement is granted there may be a damages only trial.
March 2024: To date, 1,231 lawsuits are pending, 1,398 have been filed, and no settlements have been made. Litigation continues.
August 2023: The number of lawsuits pending in the MDL expands to over 1,200.
January 2023: After a court ruling allows cases to move forward using scientific study findings, the number of lawsuits grows to over 1,000.
February 2019: Valsartan lawsuits are consolidated in a Multidistrict Litigation (MDL) in New Jersey.
Table of Contents:
What is Valsartan and How Does It Work?
Valsartan Side Effects and Injuries
Valsartan Recalls – FDA List of Recalled Medications
Eligibility to File a Valsartan Lawsuit
Recoverable Damages in the Valsartan Lawsuit
Statute of Limitations for Valsartan Lawsuits
How to File a Valsartan Lawsuit
Valsartan Payout and Settlement Amounts
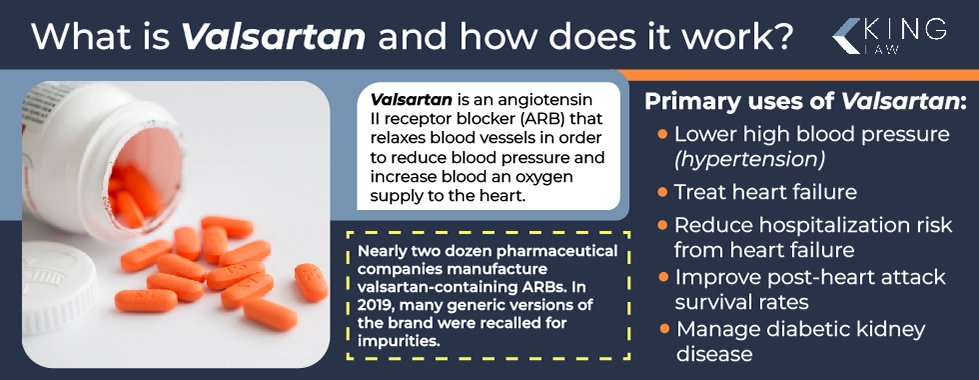
What is Valsartan and How Does It Work?
Valsartan, an angiotensin II receptor blocker (ARB) marketed by Novartis, was originally approved by the U.S. Food and Drug Administration in 1996 under the brand name Diovan. It is primarily used to treat high blood pressure, heart failure, and diabetic kidney disease. In 2012, after the Novartis’ patent expired, the market saw an expansion of generic valsartan. Most of the generic forms of the drug were produced overseas.
Valsartan-containing drugs relax blood vessels, reducing blood pressure and increasing the blood and oxygen supply to the heart. It works almost immediately, reducing blood pressure around two hours after initial intake. The drug achieves full effectiveness within two to four weeks.
There are nearly two dozen pharmaceutical companies that manufacture valsartan-containing ARBs. By 2019, many of the generic versions of the brand had been recalled by the FDA due to the discovery of unacceptable limits of impurities. This led to the FDA fast-tracking a new generic version of the drug to help mitigate shortages caused by these recalls.
What Is Valsartan Used For?
Valsartan is primarily used to reduce high blood pressure. It inhibits angiotensin II binding to the AT1 receptor, reducing vasoconstriction and aldosterone synthesis. In a short amount of time, valsartan-containing drugs facilitate easier blood flow and reduce the heart’s workload.
Primary uses of valsartan:
- Lower high blood pressure (hypertension)
- Treat heart failure
- Reduce hospitalization risk from heart failure
- Improve post-heart attack survival rates
- Manage diabetic kidney disease
The drug is often prescribed for heart and left ventricular failure after a heart attack. Millions of people have been prescribed valsartan-containing drugs, particularly generic versions of the medication.
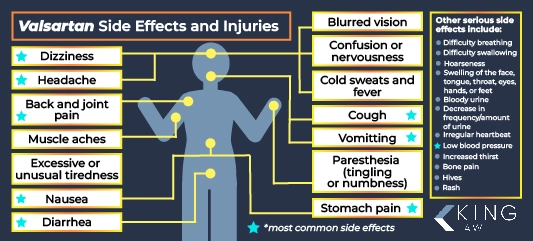
Valsartan Side Effects and Injuries
There are a number of known or reported side effects associated with taking valsartan. While some symptoms may be relatively mild, many patients have reported serious health conditions related to prolonged use of the drug.
Our law firm is accepting the following cancers as injuries for the valsartan lawsuit:
- Colorectal/colon/rectal
- Intestinal (including small intestine)
- Stomach/gastric
- Liver
- Esophageal
- Prostate
- Blood (including Non-Hodgkin’s lymphoma, Multiple myeloma, and Leukemia)
- Bladder
- Lung (Will need to know smoking history)
Potential side effects of valsartan:
- Dizziness
- Headache
- Excessive or unusual tiredness
- Diarrhea
- Nausea
- Stomach pain
- Back and joint pain
- Blurred vision
- Paresthesia (tingling or numbness)
- Muscle aches
- Cough
- Cold sweats and fever
- Confusion or nervousness
The drug may also cause serious side effects such as difficulty breathing or swallowing, hoarseness, and swelling of the face, tongue, throat, eyes, hands, or feet.
Patients taking valsartan should seek emergency medical treatment for any adverse health conditions, including bloody urine or a decrease in the frequency or amount of urine, an irregular heartbeat, low blood pressure, or increased thirst. The drug may also cause unexplained weight gain, bone pain, and hives or a rash.
Most Common Side Effects of Valsartan
The most common side effects of valsartan are typically mild and temporary but may result in discomfort, including abdominal pain, dizziness, and changes in blood pressure. Patients affected by serious or persistent side effects related to valsartan usage should consult with their doctor.
Common side effects of valsartan:
- Flu-like symptoms (cough, fatigue, joint pain)
- Abdominal pain
- Back pain
- Diarrhea
- Dizziness
- Headache
- High blood potassium (hyperkalemia)
- Low blood pressure (hypotension)
- Vomiting
Many of these symptoms are transient in nature and do not require stopping the use of valsartan. Symptoms should be discussed with a primary care physician to determine the best treatment plan moving forward.
Brand Names for Valsartan
Valsartan has been marketed under a number of name brands and generic versions of the medication. It was initially approved under the name brand Diovan in 1996. The drug is available in single-ingredient and multi-ingredient formulations.
Brand-name valsartan medications:
- Diovan (single-ingredient)
- Prexxartan (single-ingredient)
- Valturna
- Exforge
- Exforge HCT
- Diovan HCT
- Byvalson
- Vyduo
- Entresto
Currently, over two dozen manufacturers make valsartan-containing drugs in single-ingredient or multi-ingredient formulations. There are several options available for patients throughout the United States and across the globe.
Drug Companies Involved in the Valsartan Lawsuit:
By 2018, it was discovered that potentially hundreds of batches of valsartan had been contaminated with NDMA (N-nitrosodimethylamine), particularly generic versions of the drug manufactured overseas. It is alleged that Zhejiang Huahai (a Chinese pharmaceutical company) was the primary manufacturer of the contaminated lots. The drugs were then distributed by U.S. companies, including Teva Pharmaceuticals and Solco Healthcare.
Pharmaceutical companies involved in the manufacture or distribution of valsartan:
- Zhejiang Huahai (manufacturer)
- Teva Pharmaceuticals Ltd.
- Solco Healthcare
- Actavis
- A-S Medication Solutions
- AvKARE
- Bryant Ranch Prepack
- Camber Pharmaceuticals
- Hetero Labs, Inc.
- HJ Harkins Company
- Major Pharmaceuticals
- Mylan Pharmaceuticals
- Northwind Pharmaceuticals
- NuCare Pharmaceuticals
- Preferred Pharmaceuticals
- Prinston Pharmaceutical
- RemedyRepack Inc.
- Torrent Pharmaceuticals Limited
The contaminant found in valsartan is the same potentially cancer-causing toxin at the center of Zantac lawsuits. Individuals who have been exposed to NMDA as a result of taking valsartan are strongly encouraged to consult with an attorney as soon as possible.
Valsartan Mechanism of Action
Valsartan blocks angiotensin II by selectively binding to the angiotensin receptor 1 (AT1), preventing its vasoconstrictive and aldosterone-secreting effects. It provides comprehensive angiotensin II antagonism, which affects blood vessels, aldosterone production, cardiac function, and sodium reabsorption.
Unlike ACE inhibitors, valsartan prevents the action of angiotensin II regardless of its production pathway, ensuring a complete receptor blockade. It also directly acts on receptors without influencing bradykinin levels.
Ingredients in Valsartan
Diovan, a popular name-brand of the drug, contains 80 mg or 160 mg of valsartan, depending on the dosage. However, the active ingredient dosage depends on the specific brand. Most versions of the drug also contain a number of inactive ingredients that also differ by brand.
Inactive ingredients in Diovan (a name-brand version of valsartan):
- Cellulose compounds
- Crospovidone
- Gelatin
- Iron oxides
- Magnesium stearate
- Povidone
- Sodium lauryl sulfate
- Titanium dioxide
Different versions of the drug contain varying levels of the active ingredient (valsartan) and inactive ingredients.
Valsartan Recalls – FDA List of Recalled Medications
On July 13, 2015, the FDA issued a press release calling for the voluntary recall of several valsartan-containing medications following the detection of an impurity in multiple batches of the drug. The recall affected various manufacturers and was due to the discovery of a potentially carcinogenic toxin known as NDMA. Patients were advised to continue using the medication until they were able to secure a replacement product.
Manufacturers involved in the recall include:
- A-S Medication Solutions LLC
- Aurobindo Pharma
- AvKARE Inc.
- Bryant Ranch Prepack Inc.
- Hetero Labs
- Major Pharmaceuticals
- Mylan Pharmaceuticals
- Northwind Pharmaceuticals
- NuCare Pharmaceuticals
- Preferred Pharmaceuticals
- RemedyRepack Inc.
- Rising Pharmaceuticals
- Solco Healthcare LLC
- Teva Pharmaceuticals
- Torrent Pharmaceuticals
Impacted medications include those containing valsartan with amlodipine, hydrochlorothiazide, or both. Valsartan with Hydrochlorothiazide by Alembic was not affected by the recall. Some companies, including Solco Healthcare LLC, who were part of the recall, provide customer reimbursement.
Eligibility to File a Valsartan Lawsuit
Individuals must meet certain eligibility requirements to file a personal injury lawsuit. In order to determine whether you meet the criteria, you need to speak with an attorney as soon as possible.
Criteria for a Valsartan lawsuit:
- Proof of at least 6 months of valsartan use between 2015 to 2019 prior to cancer diagnosis (can be in combination with HCTZ and/or Amlodipine)
- Relevant post-2017 cancer diagnosis
- Not currently represented by an attorney
Only certain cancer diagnoses will qualify. Qualifying cancer diagnoses may include colorectal, intestinal, stomach, liver, esophageal, prostate, blood, bladder, or lung.
Evidence to Gather Before Filing a Valsartan Lawsuit:
Valsartan lawsuits are based on evidence. Therefore, it is critical to gather evidence prior to filing a lawsuit.
Evidence you may need to collect includes:
- Medical records
- Proof of Valsartan usage
- Cancer diagnosis details
- Other relevant documentation to support your case
Working with an attorney as early in the process as possible can help ensure you secure all necessary documents and information.

Recoverable Damages in the Valsartan Lawsuit
Damages in a Valsartan lawsuit are based on a number of factors, including the severity of a person’s injuries and financial losses. Compensation in these cases may include coverage of medical expenses, lost wages, and future care and treatment.
Generally, both economic and non-economic damages are available. An attorney can help ensure you receive financial recovery for all of your Valsartan-induced injuries, including your long-term care, physical pain, mental anguish, diminished quality of life, and more. Under certain circumstances, individuals who have lost a loved one may be able to file a wrongful death lawsuit on their behalf.
Statute of Limitations for Valsartan Lawsuits
Under most circumstances, there is a two-year statute of limitations on Valsartan lawsuit cases; however, the deadlines vary by state. The statute of limitations begins to toll once the injury is discovered, such as a valsartan-related cancer diagnosis. Prompt filing of a case ensures the best chance for a successful claim. Since there is no federal statute of limitations, it is imperative to work with an attorney to ensure your claim is filed timely.
How to File a Valsartan Lawsuit
If you were diagnosed with cancer after taking valsartan, you may be eligible to file a lawsuit. The first step in filing a lawsuit is to consult with an attorney. An attorney can help determine whether you have a valid claim for damages. Next, you need to understand the statute of limitations and make sure that your claim is filed timely.
Working with your attorney, you will need to gather evidence, including the necessary documents to prove your case. Finally, your legal representative will assist you in filing the appropriate paperwork to start your claim.

Valsartan Payout and Settlement Amounts
While payouts will vary depending on the severity of the injury, valsartan usage, and type of cancer, anticipated average settlements in Valsartan lawsuits are expected to be between $100,000 and $400,000.
Settlements are generally categorized into three categories based on the individual’s injury severity, drug usage, age, and cancer type. Top-tier settlements could be $400,000 or more. Middle-tier settlements will potentially be between $200,000 and $300,000. Bottom-tier payouts may be $100,000 or less. The estimated average settlement is expected to be between $150,000 and $200,000.
Contact a Valsartan Lawsuit Attorney Today
If you were diagnosed with cancer after taking valsartan, you may be eligible to take legal action. It is important to act fast to ensure compliance with all statutes of limitations and other legal deadlines. Contact King Law today to schedule a free, no-obligation consultation.