
Wegovy Lawsuit Overview
People are filing Wegovy lawsuits after experiencing severe side effects, including stomach and bowel injuries, vision loss, and blood clots. Wegovy is a semaglutide manufactured by Novo Nordisk. It was originally prescribed to treat Type 2 diabetes under the brand name Ozempic. When it was discovered that it was an effective appetite suppressant, manufacturers released a higher dose of the semaglutide to help treat obesity. Both Ozempic and Wegovy are administered via subcutaneous injection.
There have been severe health complications, including stomach paralysis related to the use of Wegovy which was approved for weight loss in 2021. Reported side effects include gastroparesis, gallbladder issues, pancreatitis, vision loss, and other serious medical conditions. If you or your loved one have experienced adverse health effects after taking Wegovy, you may be entitled to financial compensation.
Wegovy Lawsuit – 2025 Update
October 7, 2024: Novo Nordisk Faces Allegations Over Wegovy Marketing Tactics in Lawsuit
October 4, 2024: GLP-1 Drug Lawsuits Surge by 20% Amid Rising Wegovy Side Effect Claims
October 3, 2024: Novo Nordisk’s New Weight Loss Drug Amycretin Shows Promising Early Results
September 26, 2024 – Pennsylvanian Woman Sues Wegovy Manufacturer After Her Life-Altering Complications
A Pennsylvanian woman is suing Novo Nordisk following the severe health complications she experienced after taking Wegovy and Ozempic. Juanita Gantt had her large intestine removed after suffering ischemic colitis. This condition occurs when blood flow to part of the large intestine is restricted, affecting colon function. She now needs an ileostomy bag for the rest of her life. Gantt and her lawyers filed a lawsuit against Novo Nordisk, alleging the drug company did not issue proper warnings about serious complications from Wegovy and Ozempic. Her case has been consolidated into the federal lawsuit (MDL 3094 In Re: Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAS) Products Liability Litigation).
September 16, 2024 – Key Issues to Be Addressed Early in Wegovy Lawsuit by Judge Marston
Judge Marston will hear several important issues early in the Wegovy lawsuit. Wegovy Case Management Order No. 18 states that the judge will allow the defense to argue whether gastroparesis claims can be brought to court without a gastric emptying study (GES). The judge will also allow for early discovery and motion practice regarding the adequacy of the warning labels issued for Wegovy by manufacturer Novo Nordisk. Novo Nordisk claims that the FDA has approved and scrutinized the warning labels. The judge has delayed ruling on a third request—whether semaglutide is capable of causing the gastrointestinal issues alleged by plaintiffs.
September 11, 2024 – Plaintiffs Request Marketing Discovery in Wegovy Lawsuit
The plaintiffs in the Wegovy lawsuit have requested that the court issue an order permitting marketing discovery. The plaintiffs have alleged that the defendants have highly sophisticated marketing strategies to influence the medical information presented to the medical community, such as in medical journals, guidelines, and through medical organizations. Documents have shown that Novo Nordisk, the manufacturer of Wegovy, paid groups such as the American Board of Obesity Medicine. However, Novo Nordisk denies the payments are used as any form of marketing. Pharmaceutical marketing strategies often attempt to minimize the negative impacts of a drug while exaggerating its advantages. The examination of this information is going to be important in this phase of the case to prove the impacts of persuasion in the medical community and how it can mislead the patients taking the drug.
August 2, 2024 – Data Suggest a Possible Link Between Wegovy and Blood Clots, DVT, Pulmonary Embolisms
A study that investigated outcomes for people taking semaglutide drugs, like Wegovy, is getting new attention. Professionals reviewing serial trial data noted a sharp increase in a dangerous type of blood clot. The analysis published by the Endocrine Journal found a 266% increase of deep vein thrombosis (DVT) in people taking semaglutide drugs for type 2 diabetes. These clots can lead to pulmonary embolisms, which can result in death. With the use of semaglutide drugs increasing, more people may be at risk for these clots.
July 23, 2024 – Wegovy Faces Supply Shortage Amid Rising Demand and Litigation Concerns
According to the FDA Drug Shortage database, the availability of Wegovy is limited due to an increase in demand for the weight loss drug. After being FDA approved for only three years, Novo Nordisk, the parent company of Wegovy and Ozempic, has released a statement that they are seeing at least 25,000 new U.S. patients start on Wegovy every week. We expect the number of cases in this MDL to continue to steadily rise as waves of new patients begin to take the drug without Novo Nordisk’s warning of potential harmful side effects such as stomach paralysis, bowel obstruction, and blindness.
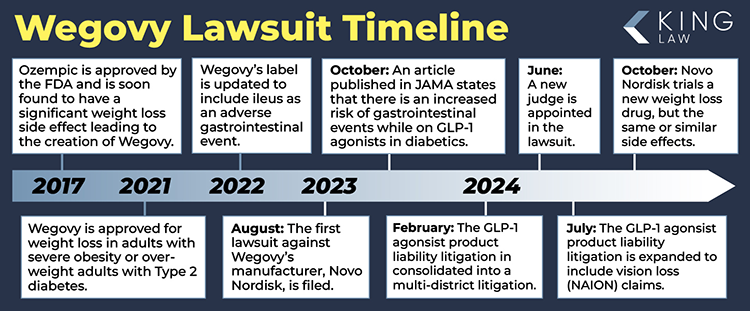
July 10, 2024 – Wegovy Lawsuit Expanded to Include Vision Loss Claims
The Wegovy Lawsuit will now include cases of vision loss. A recent study published in the Journal of the American Medical Association by Dr. Jimena Hathaway, MD, MPH, indicates that the use of Wegovy increases the risk of a certain type of blindness and vision loss. The study followed over sixteen thousand people for over six years and concluded that the use of Wegovy increased the risk of Nonarteritic Anterior Ischemic Optic Neuropathy (NAION) between four and seven times. This type of blindness, often known as NAION, is also increased by the use of Ozempic and Rybelsus, other drugs containing semaglutide produced by pharmaceutical manufacturer Novo Nordisk.
June 17, 2024 – Wegovy Lawsuit Progresses with First Status Conference Under New Judge Karen Marston
On June 10, 2024, the new judge, Karen Marston, held the first status conference in the Wegovy lawsuit since Judge Pratter’s passing. The focus was on the logistics of moving MDL 3094 forward. The judge has already issued two orders since the status conference. The first order allows lawyers who wish to file cases into the GLP-1 MDL to do so without paying a fee to be admitted pro hac vice. Secondly, the parties have filed a motion, seemingly with permission from the judge, regarding privilege logs. Parties in lawsuits are required to turn over relevant information to the other side, but sometimes there are privileges that can be asserted. For example, if a document is not turned over due to privilege, then the document must be logged, and the other side informed that it exists.
June 8, 2024 – New Judge Appointed to Wegovy Lawsuit Orders In-Person Meeting on June 10, 2024
The Wegovy Lawsuit has a new judge. Case Management Order number 7, filed on June 6, 2024, announced that Karen Spencer Marston will be the judge in the Wegovy Lawsuit moving forward. Judge Marston was a criminal prosecutor before becoming a judge. The case will now proceed, and the judge wasted no time, scheduling an in-person meeting for Monday, June 10, 2024.
May 7, 2024 – Lawyers Ordered to Present Scientific Insights on ‘Science Day’
April 11, 2024 – Wegovy Lawsuit Strategy: Novo Nordisk Files Statement of the Case
April 1, 2024 – Novo Nordisk Says Some Products for Sale Aren’t Really Wegovy, Files Lawsuit
March 20, 2024 – Wegovy Lawsuit Status Conference
The Wegovy lawsuit reached an early milestone last week. The first status conference was held in federal court in Pennsylvania. As expected, the Judge was interested to hear proposals as to how the Plaintiff’s lawyers would work together to bring what everyone expects will be thousands of cases forward. Typically, various committees will be formed with the approval of the Judge. The Judge also expressed an interest in a “Science Day” in short order. Science day will allow the Judge to learn more about the specific scientific issues surrounding Wegovy. Science day will also be a preview of some of the theories of liability by the plaintiffs and how the defense might plan to defend the case. Finally, Eli Lilly lawyers made a request for an early motion for summary judgment date and the judge did not seem inclined to grant that request. She stated that the motion to dismiss was a long time down the road.
March 17, 2024 – Judge Pratter to oversee Wegovy Lawsuit – Why it matters
The importance of the presiding judge in the Wegovy lawsuit cannot be overstated. There will be dozens of lawyers working to prove Wegovy manufacturer Novo Nordisk failed to warn of the dangers of the drug. Judge Pratter is tasked with selecting the lawyers that take on major roles, setting the schedule and ruling on the law. The case, MDL 3094 In Re: Glucagon-Like Peptide-1 Receptor Agonists Products Liability Litigation, currently includes several different drugs from several different manufacturers. We expect the number included could be reduced in the near future. Wegovy, unlike some of the other drugs is, approved for weight loss, so there will be some different legal issues for those affected by Wegovy side effects. Judge Pratter is a highly regarded federal district court Judge. She was educated at Stanford and the University of Pennsylvania Law School. She was a partner at a leading law firm and has been a federal court Judge for over twenty years. We expect the large volume of weight loss drug usage and high rates of side effects will lead to a huge volume of cases in the Wegovy lawsuit. Judge Pratter was specifically selected to oversee this lawsuit because the panel that picked her believed she was highly qualified for a huge case.
March 10, 2024 – Tracking the history of Wegovy lawsuits
The Wegovy lawsuit can trace its roots back to research by Mohit Sodhi’s team. Their work was published in Journal of the American Medical Association in fall of 2023. In that study a random sample of over 15 million people taking prescription medication showed that GLP-1 agonists cause increased risk of bowel obstruction and gastroparesis. The first Wegovy lawsuits came soon afterwards. Wegovy is approved for weight loss. It is sometimes used to treat diabetes even though it is not approved for diabetes use. Ozempic and Wegovy use the active ingredient, semaglutide. Both of these drugs will be central in the federal court lawsuit. It should be noted that Wegovy has a higher maximum dosage. Some reports indicate that higher dosages are related to more significant side effects and potential injuries. We expect that some of the most significant and permanent injuries in the GLP-1 litigation will come from Wegovy cases because of the higher doses.
March 7, 2024 – Wegovy Lawsuit takes shape
The Wegovy lawsuit is a multidistrict litigation currently pending in federal court in Philadelphia Pennsylvania. There is not a Wegovy class action lawsuit, but an MDL does have some similarities to a class action. In addition, there are also Wegovy cases pending in New Jersey state court. Lawyers like consolidation because it allows a lot of cases to be handled more efficiently. Instead of a few cases pending in every federal court in the United States all the cases involving semaglutide, a drug patented by Novo Nordisk will be heard in one court. The Judicial Panel on Multidistrict Litigation consolidated the case in February, 2024. The next important decision in the case is what firms will be involved in leading the litigation. After that is decided, there is a very important question: will the case be limited to semaglutide? ie- Ozempic Wegovy, and Rybelsus. Or, will any similar drugs be included? Our next clue is about ten days away, when the judge will hold a status conference with plaintiff and defense lawyers.
February 2024: The U.S. Judicial Panel on Multidistrict Litigation ordered the 55 cases to be combined and centralized in the Eastern District of Pennsylvania. Novo Nordisk, the manufacturer of Ozempic, also manufactures Wegovy.
January 2024: Dozens of lawsuits have been filed across the country against Ozempic, Wegovy, and Rybelsus manufacturer Novo Nordisk. The lawsuits allege that the manufacturer failed to warn consumers about the potential for dangerous side effects including stomach paralysis and gallbladder issues.
December 2023: A Louisiana federal judge allows a case against Ozempic and Wegovy manufacturer Novo Nordisk to move forward. The lawsuit indicates that the plaintiff suffered gastroparesis after taking Ozempic.
On this page:
What Is Wegovy and How Does It Work?
Wegovy Side Effects and Symptoms
Long-Term Side Effects of Wegovy Usage
Wegovy FDA-Approval and Its Black Box Warning
Eligibility Criteria for Filing a Wegovy Lawsuit
Statute of Limitations for Filing a Wegovy Lawsuit
Wegovy Settlement and Payout Amounts
What Is Wegovy and How Does It Work?
Wegovy is a prescription weight-loss medication for adults and children 12 and older with obesity or weight-related medical problems. The drug is manufactured by Novo Nordisk and administered as a weekly injection under the skin using a prefilled, single-dose pen. Individuals using Wegovy are encouraged to also follow a reduced calorie meal plan and increase their physical activity.
Individuals who are prescribed Wegovy generally must meet the following criteria:
- Adults with BMI ≥ 30 (obesity)
- Adults with BMI ≥ 27 (overweight) with a weight-related health condition
- Children 12 years and older with a BMI in the 95th percentile or higher (obesity)
Wegovy contains semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist. There is no generic version of the drug available. It is available in multiple dose strengths including 0.25mg, 0.5mg, 1mg, 1.7mg, and 2.4mg. Patients start at the lowest available dose and increase it every four weeks until they reach the maximum dose of 2.4mg.
Ozempic, which is used to treat Type 2 diabetes, is a lower dose of the same drug. Ozempic users take up to 2mg of semaglutide, compared to the full 2.4mg recommended for weight loss. It works by mimicking the glucagon-like peptide-1 (GLP-1) to reduce appetite and decrease food intake. It ultimately slows gastric emptying and prolongs feelings of fullness. The reduction in calorie intake leads to weight loss which is generally substantial in the first few months.
Wegovy Side Effects and Symptoms
Wegovy can cause several serious side effects. It is alleged that the manufacturer knew of these side effects and symptoms and failed to effectively warn consumers about the dangers. It is essential for drug manufacturers to adequately warn consumers about the dangers in order to avoid serious harm. When a manufacturer does not provide adequate warnings about a dangerous drug, they may be held legally responsible under certain circumstances.
Wegovy side effects and symptoms:
- Chronic vomiting
- Deep vein thrombosis (DVT) and pulmonary embolism (PE)
- Pancreatic cancer
- Gastroparesis (stomach paralysis)
- Severe stomach issues
- Malnutrition-related disorders
- Acute kidney injury or failure
- Liver failure
- Gallbladder issues
- Pancreatitis
- Ileus
- Inoperative pulmonary aspiration
- Esophageal injury
- Bowel or intestinal obstruction
- Increased risk of thyroid C-cell tumors
- Suicidal behavior or thinking
- Vision loss or blindness/NAION
Patients who have reported serious side effects as a result of using Wegovy have not only had concerning health complications, but they have also been left with exorbitant medical bills. Individuals who were not well-informed about the potential risks associated with taking the drug may have a valid claim for damages.
Most Common Side Effects of Wegovy
There are a number of side effects associated with taking Wegovy. Lawsuits allege that Wegovy manufacturer, Novo Nordisk, failed to warn consumers about the severity of the side effects and the potential for serious health conditions such as gastroparesis, pancreatitis, and thyroid tumors.
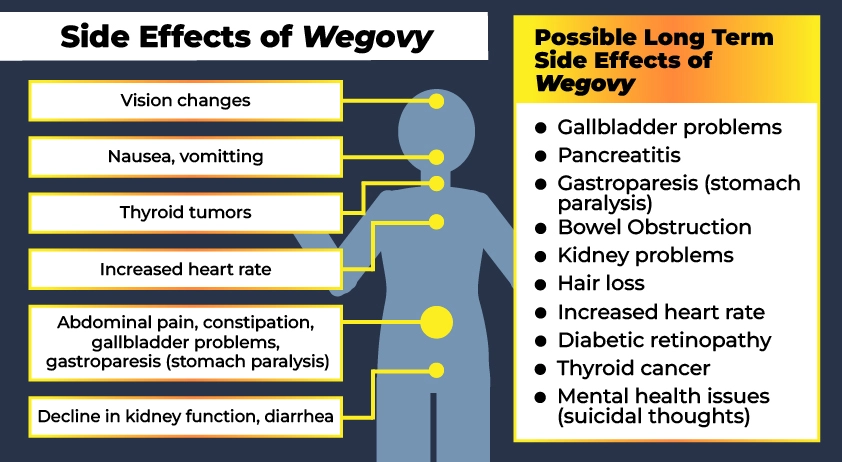
Common Wegovy side effects:
- Nausea and vomiting
- Diarrhea
- Abdominal pain and constipation
- Gastroparesis (slows or stops the movement of food from the stomach to the small intestine, also known as stomach paralysis)
- Pancreatitis
- Hypoglycemia
- Decline in kidney function
- Thyroid tumors, including cancer
- Increased heart rate and other cardiovascular effects
- Low blood sugar
- Serious allergic reactions
- Gallbladder problems
While these side effects may lessen over time, many of them can be extremely dangerous if left untreated. In some cases, the side effects of Wegovy can cause long-term health problems.
Wegovy and Vision Loss
A study released in July 2024 suggests a potential link between semaglutide drugs, such as Wegovy, and the risk of eye strokes. These eye strokes are called non-arteritic ischemic optic neuropathy (NAION). Generally, NAION is not treatable or reversible.
The study was published in JAMA Ophthalmology and had more than 16,000 participants. The findings suggest patients taking semaglutide drugs are at risk for NAION. In fact, the study suggested a three times greater risk of vision problems for patients on semaglutide drugs. The risk was similar for people taking semaglutide drugs for diabetes treatment or for weight loss.
NAION occurs when the optic nerve does not receive enough blood flow. This can lead to eye strokes that cause vision problems such as loss of vision, dark spots in the field of vision, blurring, and blindness. People who take Wegovy may be at an increased risk of these vision problems.
If you or a loved one suffered vision loss after taking Wegovy, please contact our team to discuss possible compensation from a Wegovy vision-loss lawsuit.
Wegovy and Blood Clots/DVT
When people take Wegovy, they may be at an increased risk of developing deep vein thrombosis (DVT), a type of blood clot. An analysis of an efficacy study points to this possible side effect. The analysis, published by the Endocrinology Journal, saw a 266% percent increase in DVT for people taking semaglutide drugs for their type 2 diabetes.
This finding was a result of an analysis conducted on the PIONEER and SUSTAIN serial trials, which studied the drugs effectiveness. Although the drug was found to be effective at reducing some cardiac serious adverse events (SAEs), the risk for DVT increased.
Deep vein thrombosis occurs when a blood clot forms in a deep vein within the body. This clot usually occurs in the leg. Symptoms can be minor or present as severe pain or swelling of the leg.
Wegovy and Pulmonary Embolisms
When people take Wegovy, they may be at an increased risk of pulmonary embolisms (PEs), which can be fatal. Because semaglutide drugs may put patients at risk of DVT, those patients are at risk of PEs. One of the primary causes of PE is a blood clot traveling from a larger vein to an artery in the lungs.
These lung embolisms can be very dangerous. They can cause permanent lung damage or lead to death. In fact, according to data from the National Institutes of Health, PEs caused by DVT are likely responsible for more than 100,000 deaths in America each year.
Long-Term Side Effects of Wegovy Usage
Wegovy has not only been linked to certain side effects while taking the drug, but also to long term health concerns that continue long after a person is no longer using the prescription. Signs of dangerous side effects may include abdominal pain, nausea, vomiting, and fever.
Potential long term side effects of Wegovy:
- Gallbladder problems (increased risk of gallstones and inflammation)
- Pancreatitis
- Gastroparesis (Stomach paralysis)
- Blocked intestines or bowel obstruction
- Kidney problems including acute kidney injury
- Hair loss (telogen effluvium or stress-related hair loss)
- Increased heart rate
- Diabetic retinopathy
- Thyroid cancer
- Mental health issues such as reports of suicidal thoughts
- Vision loss / changes / blindness
- Recurrent DVT
In some instances, the problem becomes so severe that surgery is required. There are ongoing studies to understand the risks associated with taking the drug, particularly related to thyroid cancer and mental health side effects. Individuals with a family history of certain thyroid cancers should avoid taking Wegovy.
Wegovy FDA-Approval and Its Black Box Warning
In June 2021, the U.S. Food and Drug Administration (FDA) approved semaglutide under the brand-name of Wegovy for chronic weight management in adults. The drug, used alongside a reduced calorie diet and increased physical activity, was the first approved drug for chronic weight management in adults with obesity or who are overweight since 2014.
Wegovy has a black box warning that notifies consumers about the potential for serious side effects such as possible thyroid tumors including cancer. Research indicates the use of Wegovy showed an increased risk of thyroid cancer in lab animals.
Uncertainty remains about whether it may have the same effect in humans. Patients with a history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia syndrome type 2 (MEN 2) are at a higher risk of developing thyroid C-cell tumors.
Wegovy Ingredient Breakdown
The ingredients in Wegovy may cause adverse effects. It is important to discuss your case with an attorney if you experienced harm after taking the prescription medication. The active ingredient in Wegovy is semaglutide which is produced using recombinant DNA technology.
Inactive ingredients in Wegovy:
- Disodium phosphate dihydrate (buffer) – 1.42 mg.
- Sodium chloride (salt) – 8.25 mg.
- Water for injection
- Hydrochloric acid or sodium hydroxide (to adjust pH)
The medication comes in a single-use injection pen without a preservative. Individuals who suffered adverse health conditions after taking Wegovy may qualify for a personal injury lawsuit.
Semaglutide Linked to Major Health Risks
Semaglutide, the active ingredient in Wegovy, has been linked to major health risks. While it helps to regulate blood sugar and aid in weight loss, there have also been reports of it causing gastroparesis, bowel obstruction, and other dangerous health conditions.
Potential risks and side effects of semaglutide:
- Stomach paralysis or gastroparesis
- Bowel obstruction
- Deep vein thrombosis (DVT)
- Malnutrition-related disorders
The risk for these side effects may increase with higher doses of semaglutide and prolonged use of the medication. Additionally, patients may be at an increased risk if they are using semaglutide specifically for weight-loss purposes and not to treat type 2 diabetes.
Wegovy Manufacturer: Novo Nordisk
Wegovy, the brand name for semaglutide, is manufactured by Novo Nordisk. Semaglutide (a GLP-1 receptor agonist) is designed to mimic a hormone that slows digestion and increases fullness. The drug was initially developed to treat Type 2 diabetes. Trials of the drug extended from January 2016 until May 2017, when the FDA approved the drug under the brand name Ozempic.
On June 4, 2021, the FDA approved the use of semaglutide for weight loss and long-term weight management under the brand name Wegovy. While both Ozempic and Wegovy are an injectable semaglutide, they differ in strength and application. Patients prescribed Ozempic to treat type 2 diabetes and help prevent cardiovascular issues are given a maximum of 2 mg of the drug, while Wegovy patients have a target dosage of 2.4 mg each week.
Some patients are unable to tolerate the higher-dose version of the drug that is recommended for weight loss. The higher dose may also lead to an increased potential for adverse effects. Nationwide lawsuits allege Novo Nordisk had insufficient warnings about the risk of harm when taking the drug, including the potential for severe gastrointestinal problems.
Eligibility Criteria for Filing a Wegovy Lawsuit
If you suffered serious side effects after taking Wegovy, you may qualify for a lawsuit. In order to file a Wegovy lawsuit, however, you must meet certain criteria and be able to provide proof of use.
Eligibility requirements for a Wegovy lawsuit:
- Proof of use: You must be able to prove that you were actively using Wegovy prior to suffering adverse health conditions. It is important to keep all medical documentation related to your prescription and subsequent injuries.
- Proof of health complications: You must be able to show that you developed a serious health complication linked directly to the use of the drug including ileus, gastroparesis, gastro-intestinal blockage, bile duct cancer, Lou Gehrig’s Disease (ALS), gallbladder cancer, vision loss, deep vein thrombosis, pulmonary embolism, or sinus cancer. This can be done through medical documentation from a doctor, ophthalmologist, or gastroenterologist.
- Proof of medical intervention: You must be able to prove that the health condition required medical intervention such as hospitalization, an emergency room (ER) visit or consultation.
It is important to note that lawsuits must be filed within a jurisdictional legal deadline, known as a statute of limitations. Legal action cannot be taken if the statute of limitations has expired. It is imperative to consult with an attorney as soon as possible to determine if you meet the eligibility requirements to file a Wegovy lawsuit.

Evidence to Gather Before Filing Your Lawsuit
Prior to filing a lawsuit, it is important to collect the appropriate evidence. Evidence may prove vital in strengthening your case. An attorney can help collect evidence and ensure that you have all of the necessary documents to prove your case.
Evidence to collect prior to filing a Wegovy lawsuit:
- Medical records
- Prescription information
- Documentation of ER visits or hospitalizations
- Doctor’s notes
An attorney can help collect evidence and ensure that you have all of the necessary documents to prove your case.
Statute of Limitations for Filing a Wegovy Lawsuit
The statute of limitations for a Wegovy lawsuit varies by state-specific laws. The best way to determine how long you have to file a lawsuit is by consulting with an attorney. A lawyer will notify you of any exceptions that may extend the amount of time you have to take legal action.
How to File a Wegovy Lawsuit
If you have suffered adverse health effects after taking Wegovy, you may be eligible to file a Wegovy lawsuit. The first step to filing a lawsuit is to contact a lawyer to schedule a free case consultation. During the initial consultation, an attorney can help determine whether you are eligible for compensation.
Next, you will need to collect medical records and other documentation to help prove your case. Evidence, including witness and expert testimony will help verify your claim for medical expenses.
The attorney you retain can then help to file the actual lawsuit and work to ensure that it meets all legal requirements including filing within the statute of limitations. Your attorney may also work to negotiate a settlement on your behalf. If a settlement cannot be reached, the attorney may set the matter for trial.
Wegovy Settlement and Payout Amounts
It is anticipated that settlements for serious injuries in Wegovy lawsuits will range from $400,000 to $700,000. The amount, however, will vary depending on the case. There is no standardized settlement amount. The severity of your injuries and the extent of your losses will determine your potential payout. Retaining an attorney can help ensure you receive the maximum compensation allowed by law.
Contact a Wegovy Lawyer to File a Claim
Were you injured after taking Wegovy? Contact King Law to schedule a free case evaluation. Our lawyers have decades of collective legal experience handling prescription drug claims. We know how to hold negligent manufacturers accountable for their wrongdoing. Contact our firm today to get started.
Frequently Asked Questions (FAQs)
Find answers to any major questions you have regarding eligibility in the Wegovy lawsuits below.

