
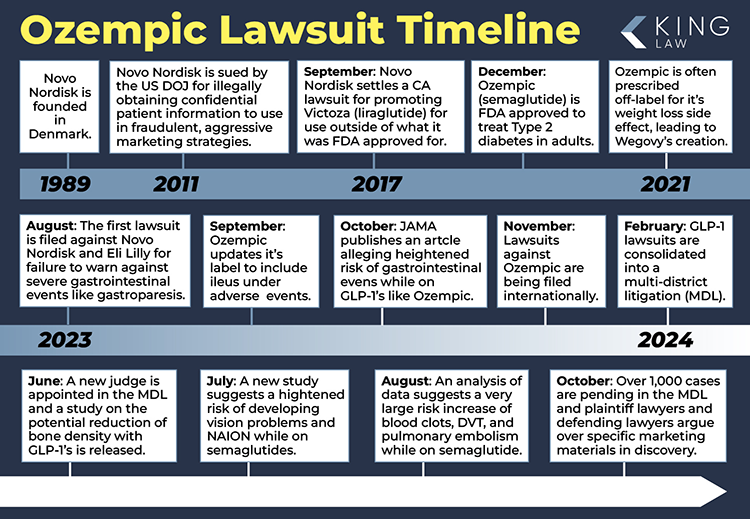
Individuals are filing Ozempic lawsuits, claiming that the drug, semaglutide, can lead to severe gastroparesis, ileus, intestinal blockages, and other gastrointestinal issues. The lawsuits allege that the manufacturer, Novo Nordisk, failed to adequately warn patients of these side effects. The first lawsuit was filed on August 2, 2023, and the number of cases continues to grow.
Ozempic Lawsuit Overview
People are filing Ozempic lawsuits after suffering from gastro-intestinal injuries, vision loss, and blood clots after taking the drug. Ozempic (semaglutide) is a GLP-1 drug approved by the U.S. Food and Drug Administration (FDA) to help treat patients with type 2 diabetes. Additionally, a well-publicized side effect of the weekly injection is that it may help people lose weight.
With increased use of the drug, concern has risen that prolonged use may lead to serious gastrointestinal injuries and other long-term issues. Our law firm is currently investigating Ozempic lawsuits involving a diagnosis of:
- Stomach Paralysis/gastroparesis
- Bowel Blockage/ileus
- Blindness (NAION)/vision problems
- Blood clots/deep vein thrombosis (DVT) or pulmonary embolism (PE)
If you have been diagnosed with any of the above conditions, or have experienced severe and prolonged vomiting, please contact us right away for a free Ozempic lawsuit evaluation. Many people harmed by Ozempic have joined the multidistrict litigation against Novo Nordisk and other GLP-1 manufacturers. This group lawsuit, called MDL 3094 In Re: Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAS) Products Liability Litigation, is an active litigation. People can still file their Ozempic lawsuit as part of this group.
Ozempic Lawsuit – 2025 Update
July 2, 2025: People Suffering Vision Loss From Ozempic May Have New Group Lawsuit Option
A group of people who suffer vision loss after taking Ozempic and Wegovy have requested that their lawsuits be consolidated in New Jersey state courts. In late June, 21 plaintiffs requested that their lawsuits be combined via a process called multicounty litigation. If the court panel approves their request, these plaintiffs will be able to bring a group lawsuit against Novo Nordisk, Ozempic’s manufacturer. If this consolidation moves forward, people who took Ozempic and developed a rare eye condition called NAION (nonarteritic anterior ischemic optic neuropathy) will have new legal filing options. People who experienced other harms from Ozempic can still join the federal group lawsuit.
June 10, 2025: Taking Ozempic Doubles a Person’s Risk of a Type of Vision Loss
The European Medical Agency (EMA) has concluded that taking Ozempic doubles a person’s risk of developing a type of vision loss called (non-arteritic anterior ischemic optic neuropathy) or NAION. The EMA, which is similar to the FDA, reviewed non-clinical studies, clinical trials, post-marketing surveillance, and the medical literature regarding NAOIN and semaglutide drugs, like Ozempic. Although the risk is relatively rare, the number of people at risk is high because of the number of people who take GLP-1 drugs containing semaglutide. NAION is a painless and sudden vision loss that is typically irreversible. It is one of the reasons people are filing lawsuits against Ozempic’s manufacturer.
June 2, 2025: Parties Will Discuss How Gastroparesis Is Diagnosed in Upcoming Ozempic Hearing
An important issue in the lawsuits against Ozempic and Novo Nordisk is how gastroparesis is diagnosed in patients. Many of the consumers who have filed lawsuits claim that Ozempic slows or stops patients’ digestion (a condition called gastroparesis). The parties have talked to different medical experts about how doctors assess whether someone has this condition, and now they will discuss their findings in an upcoming hearing. The plaintiffs’ attorneys plan to present materials about differential diagnosis, which is a standard method some medical experts use to decide if a patient has gastroparesis. This is a critical issue because many patients’ legal complaints say that the active ingredients in Ozempic caused them to develop this condition.
May 12, 2025: More Patients Sue Because of Side Effects of Ozempic and GLP-1 Products
1,809 lawsuits pending against the companies that made and sold Ozempic, the semaglutide medicine used to treat things like diabetes and obesity. Serious complications of Ozempic include gastroparesis (where the stomach empties too slowly), thyroid tumors, pancreatitis, and bowel blockages. Many patients have been hospitalized because of severe side effects. Some have lost their lives due to complications caused by the drug.
May 1, 2025: Nearly 25,000 Emergency Room Visits Attributed to Ozempic and Semaglutide Drugs
A new study found that many people end up in the emergency room due to complications from taking Ozempic and other semaglutide drugs. The study’s findings, which were published in the Annals of Internal Medicine, used data from the CDC’s NEISS-CADES project to extrapolate how many emergency room visits might be linked with semaglutide side effects. The study found that from 2022 to 2023, there were almost 25,000 visits. Most of these visits (69.3%) were due to severe gastrointestinal adverse reactions. About 37% of these visits required hospitalization. This data underscores how Ozempic and other semaglutide drugs can cause real harms to patients. Many of these patients have chosen to pursue lawsuits, claiming they were not properly warned of the dangers of these “wonder drugs.”
April 16, 2025: Expert Hearing Approaching in Federal Ozempic Lawsuit
There is an important hearing coming up in the massive multidistrict litigation against the companies that made Ozempic and other GLP-1 products. On May 14, 2025, the plaintiffs and the defendant corporations will meet to discuss a reliable diagnostic process for identifying gastroparesis. Gastroparesis, which is when someone’s gut becomes fully or partially paralyzed, is a severe complication of taking drugs like Ozempic. Discovering more details about the medical examination and treatment process for gastroparesis will help the people filing the lawsuits develop their arguments against the pharmaceutical companies.
April 1, 2025: MDL Against Ozempic and Other GLP-1s Top 1,600 in April
As of April 1, 2025, there are 1,685 active lawsuits pending against Novo Nordisk and other pharmaceutical companies that sold GLP-1 drugs like Ozempic. These drugs have been used to help people control their diabetes and lose weight in recent years. However, hundreds of consumers are experiencing severe side effects from taking Ozempic. Some people have experienced paralysis of their digestive system or lost their lives due to complications from using Ozempic. Many of these people or their surviving family members have filed lawsuits against Novo Nordisk for not properly warning them of the hazards of the drug.
March 20, 2025: People Who Filed Ozempic Lawsuits May Benefit From Two Developments in the Case
On March 18, 2025, the judge in the group Ozempic lawsuit, Judge Marston, held a court conference. During that conference, she discussed issues related to deposition protocol and the short-form complaints. Establishing the process for depositions is especially important when litigating large, corporate entities like Eli Lilly or Novo Nordisk. Attorneys representing people harmed by Ozempic will likely want more depositions, and the defense will likely want less. Plaintiffs’ attorneys want to understand what employees at these pharmaceutical companies knew, when they knew it, and how they came to know that information. Discussions about short-form complaints are promising for many plaintiffs because these complaints are much less burdensome than long-form complaints. If a short-form complaint is used, it would be accompanied by a master complaint, which would allow plaintiffs to use established facts for their case, instead of submitting these facts separately. These developments are likely a net positive for people harmed by Ozempic and other GLP-1 drugs.
March 3, 2025: Go-Forward Plan in Ozempic Lawsuits Discussed During Status Hearing
In a recent status conference for the Ozempic group lawsuit, Judge Karen Spencer Marston discussed a number of important milestones for GLP-1 lawsuits in 2025. The short-form complaint was discussed. This form will allow people harmed by Ozempic and other GLP-1 drugs to streamline documents supporting their injuries. At the conference, a date was also set for the Rule 702 hearing. This hearing, which will determine what expert testimonies will be relied on for the case, will take place on May 14, 2025. People who experienced severe digestive injuries and other serious conditions can still join the group lawsuit against GLP-1 manufacturers.
February 26, 2025: FDA Removes Ozempic From Drug Shortage List
On February 21, 2025, the FDA removed Ozempic from its drug-shortage list. Ozempic had been on this list since August 2022. The FDA received inventory and production data from Novo Nordisk, and that data made the FDA confident that the Ozempic shortage was over. Semaglutide drug compounders have already sued the FDA for this decision, saying it was a “reckless and arbitrary” decision. These events come as more people are filing lawsuits against Novo Nordisk after experiencing a range of severe side effects.
February 20, 2025: Ozempic’s Label Updated to Include Risks of Severe Injuries or Death Related to Pancreatic and Kidney Injuries
The U.S. FDA has posted an updated drug information label for Ozempic. The drug now carries a warning for severe pancreatitis and severe kidney injuries. The adverse reaction warning for pancreatitis includes fatal and non-fatal hemorrhagic or necrotizing pancreatitis. The risk for severe kidney injury is due to severe dehydration and volume depletion due to diarrhea and vomiting from Ozempic. These updates were made based on clinical trial data. Ozempic’s maker, Novo Nordisk, is already facing hundreds of lawsuits due to other severe side effects of taking semaglutide drugs.
February 3, 2025: More Than 100 New Lawsuits Join Litigation Against Ozempic and Other GLP-1 Drugs
As of February 3, 1,443 people have joined the multidistrict litigation (MDL) against Ozempic’s maker (Novo Nordisk) and other GLP-1 manufacturers. One of those who joined the MDL was a Michigan woman who says she experienced severe gastrointestinal side effects after taking Ozempic. The woman’s symptoms were so severe, she required hospitalization. Her lawsuit says Novo Nordisk did not adequately warn people of severe side effects and instead marketed Ozempic as a wonder drug. As these cases proceed through the MDL process, the next status conference will be held February 24, 2025, at 2:00 p.m. The agenda for this conference will be posted at least 3 days in advance.
January 16, 2025: Key Trial Date Set in Ozempic Lawsuits
Many people who were harmed by Ozempic have joined the consolidated federal litigation for GLP-1 drugs (MDL 3094: GLP-1 Products Liability Litigation). A key date has been set in this trial. Currently, expert witnesses on both sides are gathering information to prove and disprove that GLP-1 drugs cause many of the diseases experienced by plaintiffs. There will be an evidentiary hearing on May 14, 2025. In this hearing, the judge will determine if the reports and evidence produced by the experts can be used in court. If the opinions of the plaintiffs’ experts are deemed admissible, this will be good news for people harmed by Ozempic and other GLP-1 drugs.
January 9, 2025 – People With Blood Clots or NAION From Ozempic Will Have Separate Legal Options
Some people who have been injured by Ozempic have joined MDL 3094: In Re: Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAS) Products Liability Litigation. Recently, the Judicial Panel on Multidistrict Litigation (JPML) was asked to add two injuries to the complaint—deep vein thrombosis (blood clots) and NAION (permanent vision loss from eye strokes). The JPML decided not to add these injuries to the existing MDL. This could be positive news for people suffering from these conditions, as a new MDL could be formed. DVT and NAION are serious conditions, and people who suffer these conditions due to their Ozempic use deserve to fight for compensation that is equivalent to their injuries.
January 2, 2025: More People File Ozempic Lawsuits Ahead of 2025
As of January 2, 2025, there are 1,331 pending lawsuits against GLP-1 drug manufacturers as part of federal, consolidated litigation. People who have filed these lawsuits (plaintiffs) say they were harmed by GLP-1 drugs like Ozempic. These plaintiffs say GLP-1 drugs led them to be diagnosed with gastric disorders, liver and pancreas harms, bowel conditions, or other injuries. Many people harmed by Ozempic have joined the multidistrict litigation (MDL). From December 2024 to January 2, 2025, 31 people joined the MDL.
December 19, 2024: Two New Studies Link Ozempic to NAION, Possibly Leading to Investigation by European Medical Agency
The findings of two medical studies conducted by the University of Southern Denmark have led the Danish Medicines Agency (DMA) to ask for an investigation into the link between Ozempic use and the rare eye condition NAION. DMA will request an assessment from the European Pharmacovigilance Risk Assessment Committee (PRAC). NAION, which stands for non-arteritic anterior ischemic optic neuropathy, is an eye condition that affects the blood vessels in the eye and can lead to long-term vision loss. Both of the studies followed a large cohort of patients from Denmark and Norway who were treating their type-2 diabetes with Ozempic. The findings of both studies pointed to an increased risk of developing NAION. The DMA said the findings were strong enough that there should be an investigation into whether or not NAION should be listed as a possible adverse event when taking Ozempic.
December 18, 2024: FDA Updates Ozempic Warning Label to Include Pulmonary Aspiration as Adverse Reaction
The FDA updated the warning label for Ozempic, the GLP-1 drug used to treat diabetes and lose weight. The new label states that patients who use Ozempic and go under anesthesia or are sedated have a higher chance of getting food or liquid into their lungs, which is called aspiration. Aspiration can lead to serious complications or death. Because Ozempic causes delayed stomach emptying, patients may undergo sedation with partially digested food in their stomachs, even if they stopped eating when their physician asked them to. With the update, the FDA is urging patients taking Ozempic to talk to their doctors about the risks before being sedated.
December 12, 2024: Judge Approves Schedule for Upcoming Motions in Federal Ozempic MDL
On December 3, 2024, the federal Judge handling the massive case against the makers of Ozempic entered a scheduling order to set deadlines for the defendants’ motions to dismiss. It is common for defendants to file these types of motions. Having a set schedule for this motion gives the plaintiffs time to prepare their potential legal arguments to fight against the companies’ attempts to sidestep liability.
December 2, 2024: Ozempic and Other GLP-1 Lawsuits Reach 1,300 Active Plaintiffs
As of December 2, 2024, there are 1,300 pending lawsuits in the consolidated federal lawsuits against manufacturers of GLP-1 drugs such as Ozempic, which is manufactured by Novo Nordisk. The number of pending lawsuits in MDL 3094, Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs) Products Liability Litigation has been steadily increasing as more people allege injuries such as gastrointestinal disorders, blood clots, and vision loss were caused by their use of Ozempic.
November 27, 2024: Researchers Warn of Kidney Injury Risk When Taking Ozempic
A recent research study suggested that some patients taking GLP-1 drugs like Ozempic may experience acute kidney injuries, such as acute interstitial nephritis (AIN). People taking GLP-1 drugs may also have elevated creatinine serum levels. Creatinine is waste from muscles that healthy kidneys should be able to filter out in a person’s urine. Although GLP-1 drugs like Ozempic may have some renal benefits, the study’s authors stressed the need to monitor patients for adverse conditions like AIN and elevated creatinine serum levels.
November 26, 2024: Parties in Ozempic MDL Set Important Case Management Court Dates
On November 26, 2024, the Judge overseeing the Ozempic lawsuit entered an order setting key case management dates for the coming year. These dates allow the parties to discuss pending matters or introduce new ones. The order encourages the parties to meet before these court dates and says they can file a document suggesting agenda items up to one week beforehand.
November 22, 2024: Researchers Ramp Up Warnings About Ozempic’s Cancer Risks to Patients
Researchers continue to caution patients and medical professionals about the increased risks of using Ozempic, a glucagon-like peptide 1 (GLP-1) receptor agonist. Studies suggest a correlation between using GLP-1 drugs and an increased risk of developing thyroid cancers. The risk appears to increase with higher dosages or prolonged use of the type 2 diabetes drug. Researchers urge medical professionals to study the potential carcinogenic features of Ozempic more closely.
November 15, 2024: Plaintiffs’ Attorneys File Appearance in Ozempic Lawsuit Ahead of Expert Report Deadline
Attorneys for plaintiffs in the Ozempic MDL file their appearance before the deadline to submit expert reports supporting the injured consumers. Courts require people to file a document—called an appearance form—before they can participate in the case. The appearance form gives the Judge basic information about them, so it is on file, and the Judge knows whom to expect filings from.
November 7, 2024: Ozempic MDL Judge Sets New Deadline To File and Respond to Expert Reports
The judge handling the Ozempic multidistrict litigation (MDL 3094) signed a court order on November 5, 2024, setting critical deadlines for a round of expert reports and responses. The plaintiffs must submit their first batch of expert reports on November 18, 2024, unless they file for an extension. The defendant corporations—like Novo Nordisk—have until December 23, 2024, to file their initial expert reports.
November 1, 2024: Physicians Warn People Taking Ozempic of Skeletal Muscle Mass Loss and Call for More Research
Recent commentary published in JAMA warns that skeletal muscle mass (SMM) experienced while taking GLP-1 drugs may be of medical concern. SMM, which is called sarcopenia, likely occurs because of the rapid weight loss many people experience while taking Ozempic and other GLP-1 drugs. In their statement to JAMA, William J. Evans, PhD and Steven Cummings, MD warn that sarcopenia in older adults may be especially concerning, as it can contribute to hip fractures, disability, and increased mortality. Dr. Cummings and Dr. Evans concluded that, “There is an urgent need for studies that accurately measure muscle mass, strength, and mobility during GLP-1 agonist–induced weight loss and regain of weight after they are stopped, especially in older people.”
October 28, 2024: FDA Issues Warning About Using Compounded Semaglutide Drugs for Weight Loss
In October, the FDA issued a warning to patients who take compounded GLP-1 drugs for weight loss. The FDA said that they have received 346 reports of adverse events associated with compounded semaglutide, the active ingredient in Ozempic. Many people are taking compounded GLP-1 for weight loss. These drugs are created at pharmacies, where pharmacists combine different drugs and additives. Some estimates say up to 2 million Americans have taken compounded GLP-1 drugs, which in many cases are completely legal. In their warning statement, the FDA said taking compounded GLP-1 drugs can be risky for patients because these drugs are not evaluated by the FDA for safety, effectiveness, and quality.
October 25, 2024: More Ozempic Lawsuits Filed as People Experience Sever Side Effects
As of October, there are 1,090 pending lawsuits in the Ozempic multidistrict lawsuit (MDL -3094 IN RE: Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs) Products Liability Litigation). People who have experienced severe gastroparesis, organ injuries, blood clots, vision loss, and other complications from taking Ozempic continue to file lawsuits against Novo Nordisk and other GLP-1 manufacturers. We expect additional lawsuits to be filed in the coming months.
October 21, 2024: Judge Makes Critical Decision in Pre-Discovery Phase of Ozempic Lawsuit
The judge in the federal Ozempic lawsuit (MDL 3094) denied the plaintiffs’ motion to require the defendants to turn over specific marketing materials early in the case. One of the critical issues in the case is whether Ozempic’s warning labels gave people enough information about the potential risks and side effects of taking this weight-loss drug used to treat Type-2 diabetes.
Plaintiffs argued that the aggressive and “unprecedented” marketing campaign for Ozempic essentially drowned out the information on the product’s warning labels. They asked the judge to require the defendants to turn over marketing materials from specific periods. After each side filed their arguments and talked to the judge during several hearings, the judge denied the plaintiffs’ motion to request this information at this time.
October 17, 2024: Dispute Over Discovery in Ozempic Lawsuit Continues
Lawyers in the Ozempic lawsuit continue to debate what information Novo Nordisk should be required to provide to the plaintiffs. The defense has successfully delayed the lawsuit by arguing that the judge should first determine whether the warning label precludes certain claims, such as gastroparesis. Plaintiff lawyers have requested marketing materials that the pharmaceutical manufacturer provided to doctors who prescribed Ozempic. This is a key issue regarding what discovery will be permitted at this stage. A decision on this discovery matter is expected within a few weeks.
October 4, 2024: Large Amount of Plaintiffs File Ozempic Lawsuits in September
As of October 1, 2024, there were 1,090 cases pending in MDL 3092, Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs) Products Liability Litigation . This MDL is the Federal consolidated lawsuit against the manufacturers of GLP-1 drugs, like Ozempic. From August to September, there was nearly a 20% increase in the number of lawsuits in the MDL. The GLP-1 litigation is a fast-growing lawsuit, and we expect tens of thousands of cases to be filed as more Americans take GLP-1 agonist drugs and experience harmful, serious side effects.
October 1, 2024: Increased Reporting of Ozempic Side Effects Will Corner Novo Nordisk to Address Failure to Warn Allegations
As media outlets continue to cover more stories about the side effects of Ozempic, Novo Nordisk’s public relations team will be cornered to maintain public trust and address these claims. Despite the reported side effects from Ozempic users, ad spend for the drugmaker remains substantial and continues to drive the use of its weight loss drugs through aggressive marketing campaigns. Lately, there’s been an obvious marketing push for Wegovy in TV commercials and print ads in doctors’ offices across the country. The growing awareness of these side effects should naturally drive more interest in the Ozempic lawsuits and fuel the failure to warn allegations cast against Novo Nordisk.
September 29, 2024: Increasing Media Coverage Highlights Severe Health Risks Linked to Ozempic
Mainstream media has started covering more stories from plaintiffs alleging that the drug Ozempic caused severe gastrointestinal issues, including gastroparesis and intestinal blockage. Numerous individuals have reported unbearable stomach pains, an inability to keep down solid foods, and prolonged vomiting and diarrhea. With more people using Ozempic, we’re now seeing larger-scale reporting of the drug’s side effects. Stories about Ozempic’s health risks have routinely been overshadowed by favorable coverage about its weight-loss benefits from large media outlets.
Our firm expects media reports of gastrointestinal issues to grow, along with cases of blood clotting and vision loss in semaglutide users.
September 25, 2024: Novo Nordisk Faces Senate Scrutiny Over Ozempic Pricing and Safety Concerns
Novo Nordisk, the company that manufactures Ozempic, has recently come under fire from the United States Government for its excessive pricing, with significantly lower costs for Europeans and other countries using the same drug. This month, the CEO of Novo Nordisk, Lars Fruergaard Jorgensen, appeared before the United States Senate Committee on Health, Education, Labor, and Pensions. He reiterated that “the full value of Ozempic and Wegovy can only be realized if patients can access them. Patients need affordability and access.”
While the Senate focuses on pricing, they may be overlooking a more important issue. Novo Nordisk and the government have failed to promote awareness of Ozempic’s potential risks, including conditions such as blindness, stomach paralysis, bowel blockages, and pulmonary embolisms. Lowering the cost of Ozempic may increase the population at risk for these serious health conditions, ultimately endangering more people with its dangerous side effects.
September 10, 2024: Woman Prescribed Ozempic Files Lawsuit for Failure to Warn
In a story first reported by CBS News, Juanita Gantt shared that her doctor recommended she try Ozempic and Wegovy due to her heightened risk of diabetes and ongoing challenges with weight loss. Like many patients, she initially felt fine while taking the medications, but her health took a sudden turn when her husband found her unconscious on the floor. During her stay in the hospital, doctors found that parts of her large intestine had died and required removal. Following surgery, she suffered cardiac arrest.
Gantt is now one of thousands of individuals suing Ozempic’s manufacturer, Novo Nordisk, alleging that the drug’s warning labels fail to properly warn patients about side effects related to gastroparesis, ileus, and bowel obstruction. In response, Novo Nordisk stated that the claims against Ozempic and Wegovy are unfounded and that the company will continue to defend itself against these lawsuits brought by former users.
September 1, 2024: Kentucky Woman Files Lawsuit Alleging Ozempic Caused Gastroparesis
On August 27, 2024, a 43-year-old Kentucky woman filed an Ozempic lawsuit, alleging that the GLP-1 drugs caused her gastroparesis, or stomach paralysis. The lawsuit claims that the plaintiff used both Ozempic and Trulicity at different times from 2021 to 2024 and that the defendants (Novo Nordisk and Eli Lilly) downplayed the seriousness of the side effects and should have better warned patients.
The complaint, filed in the Eastern District of Pennsylvania federal court, alleges that gastroparesis is an incurable condition that affects normal muscle movement in the stomach and causes symptoms such as nausea, vomiting, abdominal pain, abdominal bloating, severe dehydration, and other bad side effects.
The plaintiff demands a judgment against the defendants for pain and suffering, severe and permanent personal injury, healthcare costs, and medical monitoring.
August 25, 2024: Case Management Order in Ozempic Lawsuit Favors Defense Requests for Briefing
Case Management Order No. 18 in the Ozempic lawsuit grants the defense’s request for briefing on how the injury of gastroparesis must be claimed. The defense argues that a gastric emptying test is the only objective and reasonable method of diagnosis, while the plaintiff contends that symptoms alone can serve as the basis for the diagnosis. The plaintiffs’ lawyers have previously stated that 95% of the claims in the Ozempic lawsuit will allege gastroparesis.
The defense also requested motion dates to determine whether the warning labels will preempt some or all of the claims. Additionally, the defense sought discovery and motion practice on general causation—specifically, whether GLP-1RAs can cause intestinal and bowel injuries as alleged in the Ozempic lawsuits. The judge reserved judgment on this application. So far, the judge appears to lean in favor of the defense. There are currently 842 cases pending in the Ozempic MDL.
August 13, 2024: Judge Marston Issues Cost-Sharing Order for Ozempic Lawsuit
Judge Marston has issued an order detailing how the plaintiffs’ lawyers will share the expenses incurred in the Ozempic lawsuit. Case Management Order Number 17 establishes protocols for the lawyers to submit costs during the preliminary phases of the multidistrict litigation (MDL). MDLs require dozens of law firms to work together for several years and manage the litigation. Those firms are often partially compensated through Common Benefit Funds. Money is paid into these funds by the lawyers representing individual plaintiffs. These funds help cover expenses like legal research, expert witnesses, depositions, and other shared costs necessary for advancing the case for all plaintiffs. Common Benefit Funds are funded by a percentage of the lawyer’s portion of the settlement, not the client’s portion. The goal of these funds is to ensure the costs of the broader litigation effort are fairly distributed.
August 6, 2024: Judge Marston Issues Detailed Discovery Order in Ozempic Lawsuit
Judge Marston has issued a thorough discovery order in the Ozempic Lawsuit. The 19-page order, filed on July 31, 2024, details what documents will be produced by defendant Novo Nordisk in the Ozempic Lawsuit. The order requires that the parties meet and confer on several issues. The first important issue will be what custodians the plaintiffs and defendants agree will have relevant information for the Ozempic Lawsuit. Some of the people with important documents in their possession will later be questioned under oath at a deposition by plaintiff lawyers. We believe that there will be a heavy emphasis placed on the marketing department because of the aggressive and non-traditional marketing tactics employed by Novo Nordisk in selling the weight loss drug. The lawsuit includes failure to warn claims, as well as deceptive marketing claims by the plaintiffs.
August 2, 2024: Link Between Ozempic and Blood Clots, DVT, Pulmonary Embolisms Examined
A comprehensive meta analysis that found a 266% increase of blood clots in people who use semaglutide drugs is gaining new traction. The article, published in the Endocrine Journal, looked at outcomes of patients involved in trials of semaglutide drugs. It found a substantial increased risk of deep vein thrombosis (DVT) for patients taking semaglutide drugs to treat type 2 diabetes. Patients with DVT are at risk of pulmonary embolisms, which can be fatal. As the number of people using semaglutide drugs rises, more people may be at risk for this dangerous type of blood clot.
August 1, 2024: Ozempic Lawsuit Filed by Florida Man in Pennsylvania Federal Court
A Florida man was the original plaintiff in the Ozempic lawsuit. The complaint titled Roderick Shirley v. Novo Nordisk was filed on December 15, 2023. The complaint was filed in the federal court for the Eastern District of Pennsylvania. The plaintiff alleges that the case was sued in the Pennsylvania district court because Wegovy pens are assembled there. The court document further alleges that Novo Nordisk failed to warn the plaintiff about the risks of Ozempic, demonstrating negligence and deception in marketing, and a design defect. The plaintiff alleges that he was diagnosed with deep vein thrombosis, also known as DVT, and a related pulmonary embolism when the blood clot traveled to his lungs. The injury resulted in surgery, a three-week hospitalization, and over two weeks in the ICU. The complaint alleges that the injury was the result of the use of Ozempic and Wegovy.
July 24, 2024: Plaintiffs in the Ozempic Lawsuit Required to Submit Detailed Fact Sheets
Plaintiffs in the Ozempic Lawsuit are now required to file a Plaintiff Fact Sheet. Case Management Order number 12, filed on July 14, 2024, stipulates that after filing a complaint, the plaintiff must then provide basic information to the defendant. The fact sheet requires the plaintiff to specify the type of GLP-1 RA they used and the injuries they allegedly suffered. The plaintiff must also state whether they are alleging economic damages or psychological injury. Plaintiff fact sheets are common requirements in multidistrict litigations. This is an important step in the lawsuit. For plaintiffs interested, we have access to the detailed, 17-page Ozempic Plaintiff Fact Sheet provided by the judge for viewing.
July 23, 2024: Rise in Ozempic Lawsuits Following Study on Vision Damage Risks
Ozempic lawsuits have been on the rise, especially since the publication of a previous study showing an increased risk for sudden vision damage. Allegations continue to surface that Ozempic can lead to multiple life-long conditions such as gastroparesis and Nonarteritic Anterior Ischemic Optic Neuropathy (NAION), dramatically impacting lives. We are still accepting cases for this litigation.
July 9, 2024: New Study Links Semaglutide to Increased Risk of Vision Problems
A new study by Dr. Jimena Hathaway, MD, MPH, published in the Journal of the American Medical Association, indicates that Semaglutide, the active ingredient in Ozempic, Wegovy, and Rybelsus, shows an increased risk of vision loss. The study found that in a pool of 16,827 patients, Nonarteritic Anterior Ischemic Optic Neuropathy (NAION) increased more than four times for people taking semaglutide compared to those who took another drug. The analysis was conducted from 2017 to 2023. This is a critical study in the Ozempic lawsuit because the label does not warn of this side effect. Eye doctors and lawyers have suspected vision problems for some time. We expect that the federal court lawsuit will soon include Ozempic vision loss cases, including blindness and blurred vision.
June 28, 2024: Study Reveals Potential Bone Density Reduction with GLP-1 Treatment
In a recent study, Simon Jensen, Ph.D., and others found that GLP-1 treatment, without exercise, could lead to reduced bone density. The study was published on June 25, 2024, in JAMA Network. The most common GLP-1 is Ozempic. The study follows speculation that the reduction in nutrient and water absorption by the body could have long-term health implications. So far, the Ozempic lawsuit has focused on gastrointestinal side effects like gastroparesis and bowel obstructions.
June 17, 2024: Ozempic Lawsuit Progresses with New Judicial Orders and Status Conference
The Ozempic lawsuit is again beginning to move. The first status conference with the new judge was held on June 10, 2024. The focus was on the logistics of moving the MDL forward. Since the conference, the judge has issued two mundane orders. First, lawyers who wish to file cases into the GLP-1 MDL will not have to pay a fee to be admitted pro hac vice. Second, the parties have filed a motion, seemingly with permission from the judge, in regards to privilege logs. Parties in lawsuits have to turn over relevant information to the other side in lawsuits like this one. However, sometimes there are privileges that can be asserted. If a document is not turned over due to privilege (like a communication with a lawyer), then the document is supposed to be logged and the other side informed that it exists. This log is called a privilege log and is part of every case.
June 8, 2024: New Judge Appointed in Ozempic Lawsuit, Case Moves Forward
There is a new judge in the Ozempic Lawsuit. Case Management Order number 7 was filed on June 6, 2024, by Judge Karen Spencer Marston. Judge Marston will be the new judge overseeing the case. She was nominated for the federal bench following a lengthy career as an Assistant US Attorney. The next conference will be held in person in the Eastern District of Pennsylvania on June 10, 2024.
June 2, 2024: Ozempic Lawsuit Paused in Federal Court Awaiting New Judge Appointment
The Ozempic lawsuit pending in federal court is at a standstill until a new judge is appointed following the unexpected death of the previous judge. We also expect to see cases continue to be filed in New Jersey state court. Federal court is the appropriate venue when there are parties from two different states. Cases that will be brought against Novo Nordisk by residents of the State of New Jersey will probably have to be brought in New Jersey court. It is possible that other plaintiffs will file claims in New Jersey state court as well.
May 22, 2024: Science Day Canceled in Ozempic Lawsuit, Case Reassignment Possible
Science Day has been canceled in the Ozempic Lawsuit. The Chief Judge of the Eastern District of Pennsylvania, Mitchell Goldberg, issued an order on May 21, 2024, vacating Case Management Order number 5 and canceling Science Day. The case must be reassigned. Perhaps Judge Goldberg will be the new judge on the case.
May 20, 2024: Judicial Shift in Ozempic Lawsuit After Judge Pratter’s Passing Impacts Proceedings
The Ozempic lawsuit is in a period of flux with the death of Judge Pratter. Judge Pratter was the presiding Judge in the lawsuit and was taking an active and aggressive role. The plaintiffs and defendants will now await a new judge to be appointed by the Judicial Panel on Multidistrict litigation. Plaintiffs will hope for a more liberal plaintiff friendly judge. The Eastern District of Pennsylvania is thought to be pro plaintiff, with several large verdicts in Philadelphia recently. The death of Judge Pratter may delay the case temporarily.
May 19, 2024: Novo Nordisk and Eli Lilly Outline Legal Strategy to Reduce Liability and Challenge Plaintiff Claims on Ozempic and Mounjaro
Novo Nordisk and Eli Lilly recently outlined their strategy in a defendant statement to reduce liability and minimize plaintiff recoveries. Both companies covered the benefits and advantages of Ozempic and Mounjaro and countered the plaintiffs’ focus on failure to warn for GLP-1RAs. The defense categorized the lawsuits into different injury classes which puts pressure on the plaintiffs to provide specific evidence for each of the cases. They’re also pushing for plaintiffs to disclose information about unfiled cases, but that is not legally required. They also pushed for early motions to dismiss by requesting objective testing for “gastroparesis,” which can complicate the plaintiffs’ burden of proof.
May 3, 2024: Plaintiffs Respond to Defendants’ Position Statement in Ozempic Lawsuit
The plaintiffs filed a statement in the Ozempic lawsuit responding to the defendants’ “Statement of the Case”. The plaintiffs’ statement outlines the framework they will argue and attempts to dismiss the defendants’ arguments from their filing. The statement highlights a conflict between the two parties about what should be required to file a lawsuit. The defendant has argued that a plaintiff should be required to have a gastric emptying study to bring a lawsuit. The plaintiffs’ lawyers say that the test is not required. Instead, they assert that a doctor’s diagnosis of gastroparesis, stomach paralysis, or bowel injuries is sufficient, at least for the early stages of a lawsuit.
April 30, 2024: ‘Science Day’ Scheduled for the Ozempic Lawsuit
Judge Pratter issued Case Management Order number 5 in the Ozempic lawsuit and scheduled “Science Day” for June 14, 2024. Science Day will provide an overview of the medical and scientific issues relevant to the case. Plaintiffs and Defendants will have a designated time slot to present their positions to the Judge during Science Day. It would be interesting to know whether the plaintiffs’ lawyers will focus on Ozempic and other semaglutide drugs or split their time with some of the Eli Lilly drugs as well. Unfortunately, the Judge ruled that the record from Science Day will be confidential to the Court.
April 21, 2024: Drawing Parallels – Ozempic Lawsuit and Benicar MDL Settlement Analysis
Some guidance in the Ozempic lawsuit might be gained from a review of the Benicar MDL. Benicar was a common blood pressure medication in the early 2000s. Like the Ozempic case, the Benicar case alleged a failure to warn of serious gastrointestinal side effects, like chronic diarrhea and bowel obstructions. In 2017, the case settled for a reported $300 million to 2,300 plaintiffs. Other reports indicated the actual settlement value was closer to $360 million. The most serious cases are reported to have settled for over $500,000, while others received much less. The average, however, seems to have been well over $100,000. It would not surprise us if the settlement matrix in that case is a target for the lawyers in the Ozempic case.
April 20, 2024: Ozempic Lawsuit Is About Whether Users Knew of Potential Side Effects
The plaintiffs and defense have begun to submit their respective positions to the judge in the Ozempic lawsuit. Early court documents show that this case will be about whether the manufacturer properly warned of the side effects. Plaintiff lawyers say that gastroparesis and other bowel injuries are unwarned consequences of the use of Ozempic and similar drugs. The defense states that intestinal issues, some severe, are side effects that everyone knew about. Part of their defense is that the doctors knew of the side effects and had an obligation to inform the patients.
April 11, 2024: Ozempic Lawsuit Joint Defense Statement Filed by Novo Nordisk and Eli Lilly
Defendants Novo Nordisk and Eli Lilly have submitted a joint statement of the case in the ongoing Ozempic lawsuit. This document, filed in federal court on April 9, 2024, aims to outline the defense’s strategy over the coming months. At the time of filing, 117 plaintiffs had initiated lawsuits: 100 cases targeted Novo Nordisk, 8 targeted Eli Lilly, and 9 involved both defendants. According to the defense, GLP-1RA drugs are revolutionary medications with FDA-approved warnings, asserting that they are generally safe and have undergone extensive testing. The defense also claims to have insufficient information about both filed and potential cases. They note a rise in counterfeit products and assert that many plaintiffs alleging “gastroparesis” lack substantial evidence of their diagnosis. This filing provides a significant insight into the defenses planned for the Ozempic lawsuit.
April 10, 2024: Additional Cases Transferred to Eastern District of Pennsylvania
The April 4, 2024, conditional transfer order signed by Tiffaney D. Pete, Clerk of the Panel on Multidistrict Litigation, states that two additional cases were transferred to the United States District Court for the Eastern District of Pennsylvania and have been assigned to Judge Gene Pratter as part of the Ozempic Lawsuit (MDL No. 3094). The Order notes that 17 cases were transferred in the original transfer order on February 2, 2024. Since that time, 36 more cases have been added to the lawsuit. Like most MDLs, the filings have been slow to start with. Most law firms will wait for the lawyers to agree on a short-form complaint, then file their cases. A short-form complaint is a simplified pleading that gives the basics of each plaintiff’s complaint. We expect that the Ozempic complaint will include things like which drug was prescribed to the plaintiff, date of prescription, the diagnosed injury, the theory of liability against, and whether the claim is against Novo Nordisk or Eli Lilly.
April 1, 2024: Ozempic Manufacturer Novo Nordisk Sues Pharmacies for Knock-Offs
Ozempic manufacturer Novo Nordisk continues to pursue lawsuits against pharmacies that sell compounded semaglutide. The FDA has discouraged the National Association of Boards of Pharmacy from compounding Ozempic, Wegovy, and Rybelsus. The FDA further notes that different forms of semaglutide appear to violate federal law. On January 10, 2024, the FDA issued guidance acknowledging the risk and popularity of knock-off Ozempic. The biggest concern is “compounded” Ozempic. Novo Nordisk has settled several lawsuits against pharmacies providing substances that are not, in fact, Ozempic. Of course, the federal lawsuit alleges that even in its purest form, Ozempic can be dangerous, and the manufacturer is now properly warning of the risks associated with the drug.
March 23, 2024: Ozempic Lawsuit Leadership Structure Proposed
Plaintiff lawyers in the Ozempic lawsuit have submitted a motion for leadership structure to the court. This is no surprise. Court leadership needs to get to work on setting schedules and making requests for documents from the drug manufacturers Novo Nordisk and Lilly, formerly known as Eli Lilly. Our litigation partner Daniel Nigh, Esq. is a proposed member of the executive committee. Daniel is an experienced dangerous drug lawyer and we expect him to be on the front lines of the litigation of the serious scientific issues in the Ozempic case. We expect the motion to be granted in short order by Judge Pratter.
March 21, 2024: Anticipating a Surge of Ozempic Lawsuits in New Jersey Courts
The Ozempic lawsuit will soon become the Ozempic lawsuits. We believe that a substantial number of Ozempic lawsuits will be filed in New Jersey state court, in addition to the cases being filed in federal court. We expect that law firms with a large volume of Ozempic cases will file some in state court as a way to hedge their bets against putting all their cases into one litigation, in front of one judge. The biggest risk in a large federal court tort case is a bad Daubert ruling. “Daubert” refers to a landmark case that sets the standard for what an expert can testify to. A bad Daubert ruling can lead to the dismissal of all cases. New Jersey is the most likely state court venue because it is the U.S. headquarters of Novo Nordisk, the Ozempic manufacturer. In addition, some lawyers who are not satisfied with their role in the federal court case may choose to file in state court instead. Several Ozempic cases have already been filed in New Jersey state court.
March 20, 2024: Status Conference Update in Ozempic Lawsuit
The first status conference was held in the Ozempic Lawsuit last Thursday. The conference began with the Judge asking for proposals about how the plaintiffs would organize the several lawyers from law firms around the country who are filing cases. Lawyers from Eli Lilly made an aggressive early play asking the judge when they could file for summary judgement. The court said that would be a long time down the road. Early estimates are that Eli Lilly will defend about 10 percent of the cases for their drug, Mounjaro. Some lawyers believe that the most serious injuries come from Ozempic and Wegovy. The comments seem to indicate that the case will include both manufacturers for the foreseeable future. Finally, the court indicated a “science day” where each side gets to present to the Judge about the science involved in this case. Science days help the Judge understand the case more fully as they rule on legal issues. We anticipate science day will occur in the next three months in the Ozempic Lawsuit.
March 10, 2024: IMPORTANT DECISIONS UPCOMING IN OZEMPIC LAWSUIT
Judge Gene E.K. Pratter, the presiding Judge in the Ozempic lawsuit, will soon appoint lawyers to certain roles and set a discovery schedule. She will also make decisions about what cases are included in the lawsuit and what evidence is allowed to be used at trial. Judge Pratter might be considered a blue-blood judge. She graduated from Stanford and received her law degree from the University of Pennsylvania. The University of Pennsylvania law school is currently ranked #4 in the United States. She was a partner in the law firm of Duane Morris from 1975, until her nomination to the federal bench by George W. Bush. She was confirmed by the Senate on June 15, 2004. She is a member of the adjunct faculty at the University of Pennsylvania. Her resume is that of an experienced and intelligent judge. We expect the Ozempic lawsuit to be one of the largest and most complex in the history of the United States. It is no mistake that the case was assigned to Judge Pratter. Local attorneys know her as a no-nonsense type. We expect Judge Pratter to be impressive this week at her first status conference in the Ozempic litigation and show the lawyers that she is going to run a tight ship.
March 2, 2024: Upcoming Ozempic Lawsuit News
There hasn’t been much news in the Ozempic lawsuit since it became official and was consolidated in the Eastern District of PA. We’ll get our initial look at how the Ozempic lawsuit is shaping up at the first status conference scheduled with judge Gene E.K. Pratter on March 14, 2024. We continue to see Ozempic everywhere on TV and social media. You can’t go very long without being fed its so-called greatness on mainstream news or the countless influencers on Facebook, Instagram, and others. While it is portrayed as a miracle weight loss drug, there is the dark side of it’s serious side effects like stomach paralysis affecting thousands of people.
February 28, 2024: Behind the scenes of the Ozempic Lawsuit
The Ozempic lawsuit is in the critical early stages of the Multidistrict Litigation. The politics of mass tort litigation is difficult to understand. Simply put, there are different factions in almost every case. Law firms want power and authority to control the decisions in the case because they have a lot of resources invested in getting the case to become an actual lawsuit. The Ozempic lawsuit highlights the differences between some of the “old guard” lawyers and some of the big firms that are entering mass torts. One big firm filed for consolidation of the Ozempic lawsuit, allegedly without much warning to other law firms. Other lawyers then were forced to rush to get cases filed and have the right to be heard at the JPML hearing. The responding lawyers asked for the case to be transferred to Philadelphia – and the panel granted their request. There is some inherent conflict between law firms and at this point we will see if the different factions can work together to propose a group of lawyers to drive the case forward for the next several years. The judge will appoint a lead counsel and plaintiffs leadership committee in the next several months. The group will then proceed with discovery and learn how Novo Nordisk developed Ozempic, Wegovy, and Rybelsus – and most importantly what they knew about the risks when they failed to warn of stomach paralysis and intestinal blockage.
February 24, 2024: Ozempic manufacturer files lawsuits targeting knockoffs
Ozempic Manufacturer Novo Nordisk is not only being sued in federal and state courts for injuries caused by semaglutide, the official name of the drug contained in Ozempic, Wegovy and Rybelsus they are filing their own lawsuits too. Novo Nordisk owns the patent for semaglutide and no other manufacturer is allowed to produce it. The company has filed at least 12 lawsuits against entities they say are selling knockoff Ozempic. Novo Nordisk has aggressively targeted pharmacies and online drug sellers for “compounding.” Novo Nordisk maintains that they are the only company allowed to sell semaglutide and allege that they do not know what is contained in the compounded drugs being sold online. The company states the compounded drugs are not FDA approved.
February 23, 2024: Ozempic Manufacturer Has faced many lawsuits
Ozempic manufacturer Novo Nordisk is no stranger to lawsuits. The company has recently been in the news because Ozempic lawsuits are popping up everywhere. Earlier this month the federal court cases were centralized in Philadelphia for efficiency. A new case filed in New Jersey State Court, Tinsley v. Novo Nordisk, also suggests that some lawyers think it will be advantageous to stay outside the class action style lawsuit and proceed with a New Jersey state court Ozempic case. It is worth noting that the New Jersey case is a gallbladder case and we believe gallbladder cases will not be part of the federal court lawsuit.
It should be noted that the United States Department of Justice filed a lawsuit against Novo Nordisk nearly 15 years ago for fraudulently and aggressively marketing their diabetes drugs. Novo Nordisk Settled that case brought by Loretta Lynch, United States Attorney for the Eastern District of New York. In 2017 the State of California settled with the Ozempic manufacturer for promoting a type two diabetes drug outside of what it was approved to be used for. In that case the company targeted children for the diabetes drug, even though the FDA had not approved the drug for use in children. Similarly, the current lawsuit alleges that Novo Nordisk aggressively marketed Ozempic using inappropriate tactics and has promoted a diabetes drug for a use inconsistent with FDA approval.
February 21, 2024: Ozempic Manufacturer Failed to Warn of health risks
The Ozempic lawsuit will be largely focused on what the manufacturer knew about the side effects of using Ozempic, and when. Plaintiffs’ lawyers have brought the lawsuit so they will have the burden to prove that Novo Nordisk did something wrong. The most likely claim to be successful is a failure to warn of the dangers associated with the drug. Many lawsuits have been filed alleging that Ozempic the manufacturer knew of the risk of gastroparesis, ileus and bowel obstruction. The allegations also include aggressive and deceptive marketing. On October 5, 2023, an important study, published in JAMA and authored by Mohit Sodhi, indicated an increased risk of gastroparesis, bowel obstruction, and pancreatitis for those using GLP-1 agonists. Ozempic is the trade name for semaglutide. The study used a cohort of over 5,000 randomly selected individuals from the PharMetrics Plus for Academics Database of over 16 million prescription drug users in the United States. The Ozempic lawsuits began being filed around the United States shortly after this study. By December an application was made to consolidate all Ozempic lawsuits in a federal class action type, Multidistrict Litigation. The application was granted, and we are in the early stages of the Ozempic MDL, centered in Philadelphia. The first status conference before the Judge is forthcoming. The first milestones in the case will be selecting lead counsel, filing a master complaint and establishing a preliminary discovery schedule for document production.
February 16, 2024: First Status Conference set in Ozempic lawsuit.
Just a week after the Judicial Panel on Multidistrict Litigation created MDL 3094 (the official Ozempic Lawsuit) and transferred all cases to the Eastern District of Pennsylvania, the Hon. Gene E.K. Pratter has scheduled the first status conference for Thursday, March 14, 2024. Among the topics to be discussed are: Organization and Process for selecting plaintiff’s lead counsel, scheduling and frequency of future status conferences, filing procedures for motions, responses, complaints, and other documents, and the creation of plaintiff fact sheets. Perhaps the most important agenda item is discussion on whether to keep this just an Ozempic case, or to include other manufacturers such as Rybelsus, Wegovy, Trulicity, and Mounjaro.
February 15, 2024: Ozempic Lawsuit Injuries Coming Into Focus
With the Ozempic lawsuit just weeks old, we are refining which injuries will likely be strong cases. It is important to understand that the basis of the lawsuit is the failure to warn about the potential for serious side effects caused by using Ozempic and similar drugs. There is solid support for gastroparesis, stomach paralysis, gastrointestinal obstruction, and ileus (bowel obstruction).
With respect to gallbladder cases, most people with these issues will not be eligible in the federal MDL lawsuit. This is because Novo Nordisk, the maker of Ozempic, added a gallbladder warning in March of 2022. This effectively disqualifies anyone with gallbladder complications who took the drug after March 2022. It could also disqualify anyone who took the drug prior to the date of the warning because most states have a two-year statute of limitations to file a lawsuit.
February 4, 2024: Ozempic Lawsuit becomes official
There is now an active Ozempic Multidistrict Litigation, as we expected. This is similar to an Ozempic Class Action Lawsuit. The case will be known as MDL 3094 IN RE: GLUCAGON-LIKE PEPTIDE-1 RECEPTOR AGONISTS (GLP-1 RAS) PRODUCTS LIABILITY LITIGATION. The case will be heard in the Eastern District of Pennsylvania federal court. Hon. Gene E. K. Pratter will be the Judge. This venue is generally considered a plaintiff-friendly venue. There have recently been large verdicts in the Philadelphia area against Monsanto in the Roundup litigation. The other big question that was left open during the January 25th hearing was who would be included in the case. Eli Lilly, the manufacturer of Mounjaro, has been included in the Ozempic federal court lawsuit. Eli Lilly has done their best to distance themselves from Novo Nordisk. The Judicial Panel on Multidistrict Litigation (JPML) seems to have been conflicted on whether or not to include them. The order specifically states that if Judge Pratter decides it is not a good decision he has options to separate the defendants. For now, however, Mounjaro is part of the Ozempic lawsuit. The first important question for Judge Pratter will be, who is going to lead this litigation for the plaintiffs’ side.
February 1, 2024: Decision on the Future of Ozempic Lawsuit Looms
The Judicial Panel on Multidistrict Litigation is set to rule on how the Ozempic Lawsuit will proceed. At hand remains the question as to whether the lawsuit will move forward to include only the Ozempic brand itself, which is manufactured by Novo Nordisk, or if other similar drugs such as Ely Lilly’s Mounjaro will become part of the suit. A decision from the JPML is expected in February.
January 25, 2024: Pivotal Day For Ozempic Lawsuit
The Ozempic lawsuit is at a critical stage today. The Judicial Panel on Multidistrict Litigation will hear arguments in Santa Barbara, CA, with respect to the application brought by plaintiffs to create a multidistrict litigation. It is nearly certain that they will grant the application because the Ozempic manufacturer does not object. There are two questions remaining, however. First, will drug manufacturer Ely Lilly be included in this case. They make the drug Mounjaro, which is similar but different from Ozempic. Some lawyers think it is better to keep them separate. Finally, where will the case be located? The application requested the Western District of Louisiana. There are also applications for the lawsuit to be based in federal district court in California, North Carolina, and Philadelphia.
January 20, 2024: Manufacturers of Ozempic and Mounjaro take differing positions in Lawsuit
The future of the Ozempic/Mounjaro litigation is still undecided. After an application by plaintiffs to organize the case in a Multidistrict Litigation (MDL), the two largest defendants Novo Nordisk and Eli Lilly are split on their response to the application. Novo Nordisk, the manufacturer of Ozempic, has supported the application for consolidation but requests the case be heard in the Middle District of North Carolina or the Southern District of California. The Ozempic response was filed with the Panel on December 29, 2023. The different venues here are surprising. The middle of North Carolina is probably a play at a lower average income jury pool, as a way to limit exposure and perhaps a move towards more conservative jurors that would not be as likely to support drug use for weight loss. California on the other hand seems like a risky request. California verdicts can be some of the highest in the country. The defense must feel that the jury pool would think that it was common knowledge there were risks associated with the use of Ozempic.
Eli Lilly, the pharma giant and manufacturer of Mounjaro has asked the JPML to deny the plaintiffs’ motion for consolidation of the lawsuits. The Ozempic manufacturer probably thinks it will be too difficult and expensive to defend cases in virtually every federal court in America. The Mounjaro manufacturer is likely trying to distance themselves from Ozempic, which has been largely used and abused off label.
January 14, 2024: Application Made to organize Ozempic/Mounjaro lawsuits into a “class action” style MDL
A recent application by attorneys of those injured by the side effects of drugs like Ozempic and Mounjaro to the Judicial Panel for Multidistrict Litigation (JPML), asks the panel of Judges to consider referring all Ozempic and similar lawsuits nationwide into a single federal district court. The benefits of consolidation into a Multidistrict Litigation (MDL) include efficiency in the pre-trial discovery process, cost effectiveness for all parties, and fairness among all plaintiffs with similar injuries. An Ozempic MDL has similarities to an Ozempic Class Action Lawsuit, although the main benefit for plaintiffs is that an MDL allows for individualized settlements based upon the severity of each person’s case. The application includes the drugs Liraglutide, Dulaglutide, Semaglutide, and Tirzepatide. Those drugs are marketed as Saxenda, Trulicity, Ozempic, Rybelsus, Wegovy, and Mounjaro. King Law is currently investigating cases where individuals used these drugs and were further diagnosed with gastroparesis, stomach paralysis, gastrointestinal obstruction, and ileus.
January 7, 2024: Ozempic lawsuits increase in frequency following a recent study
Published in The Journal of the American Medical Association (JAMA), the study found Ozempic and other drugs with Glucagon-Like Peptide-1 Receptor Agonists are linked to pancreatitis, bowel obstruction, and gastroparesis. The study by Mohit Sodhi, and colleagues, looked at a random sample of 16 million patients from 2006 to 2020. Ozempic lawsuits are popping up all over the country. The general claims are that Ozempic was not properly studied before its release, there were not proper warnings about the effects of Ozempic, and Ozempic is being used off label. Off-label use of medical devices and drugs like Ozempic is a common allegation in a lawsuit. Drugs are studied and approved for certain uses. In the case of Ozempic, the medication is approved for treatment of diabetes. We anticipate much of the Ozempic litigation will center around the marketing practices that encourage use for weight loss, instead of what the FDA said the drug could be safely used for.
November 18, 2023: Ozempic Lawsuit Allegations Grow Internationally
In addition to a possible class action type lawsuit in the United States against the manufacturers of the Diabetes drug Ozempic, a Canadian law firm has now filed a similar suit in British Columbia. The lawsuit alleges the same type of failure to warn of side effects such as Gastroparesis, Stomach Paralysis, Ileus, Gallbladder issues, and Gastric Intestinal Obstruction.
November 18, 2023: Ozempic Class Action MDL Lawsuit Could Come Summer 2024
A Class Action lawsuit could come as early as summer 2024 against the manufacturer of the popular drug Ozempic. The allegations are that the drug, which helps regulate type two diabetes and assist in weight loss, causes severe gastrointestinal problems. Those who have experienced these injuries claim that pharmaceutical companies Novo Nordisk and Eli Lilly failed to warn patients of the adverse side effects from Ozempic and its generic sister drug Semaglutide.
October 2023:
A research letter filed in the Journal of the American Medical Association alleges the risk of adverse gastrointestinal events associated with the use of GLP-1 agonists that are used not only to treat diabetes but also off-label for weight loss. The research concludes that there is an increased risk of pancreatitis, gastroparesis, and bowel obstruction with the use of the drugs.
August 2023:
The first lawsuit is filed against the makers of Ozempic and its sister drug Wegovy alleging a failure to adequately warn consumers about the potential risk of harm associated with using the drugs. The Ozempic lawsuit, filed against pharmaceutical giants Novo Nordisk and Eli Lilly, alleged that the drugs, both Glucagon-like peptide 1 (GLP-1) agonists, caused severe gastrointestinal issues.
On this page:
What Is Ozempic and How Does It Work?
Ozempic Side Effects and Health Risks
Ozempic Manufacturer: Novo Nordisk
Eligibility Criteria for Filing an Ozempic Lawsuit
How to File an Ozempic Lawsuit
What Is Ozempic and How Does It Work?
What is Ozempic?
In December 2017, Novo Nordisk received approval from the U.S. Food and Drug Administration (FDA) for the use of their drug Ozempic (semaglutide) as a weekly injectable to help treat adults with type 2 diabetes. Ozempic is a weekly injectable that helps to lower blood sugar and reduce the risk of heart attack and stroke in people with type 2 diabetes and heart disease.
Though the drug was not initially approved for weight loss, it is increasingly prescribed off-label for this reason since weight loss is a common side effect. Wegovy, a higher dose injectable of the same drug, was approved by the FDA for weight management in 2021.
How does Ozempic work?
Ozempic and its sister drug Wegovy, are GLP-1 receptor agonists. GLP-1 receptor agonists work by slowing digestion through the release of a hormone that not only aids in insulin release but also blocks glucose production. It mimics a naturally occurring hormone that sends a signal to the brain when you are full, ultimately slowing digestion. It is intended for long-term use. Discontinuation of the drug can lead to the regaining of the weight that was lost.

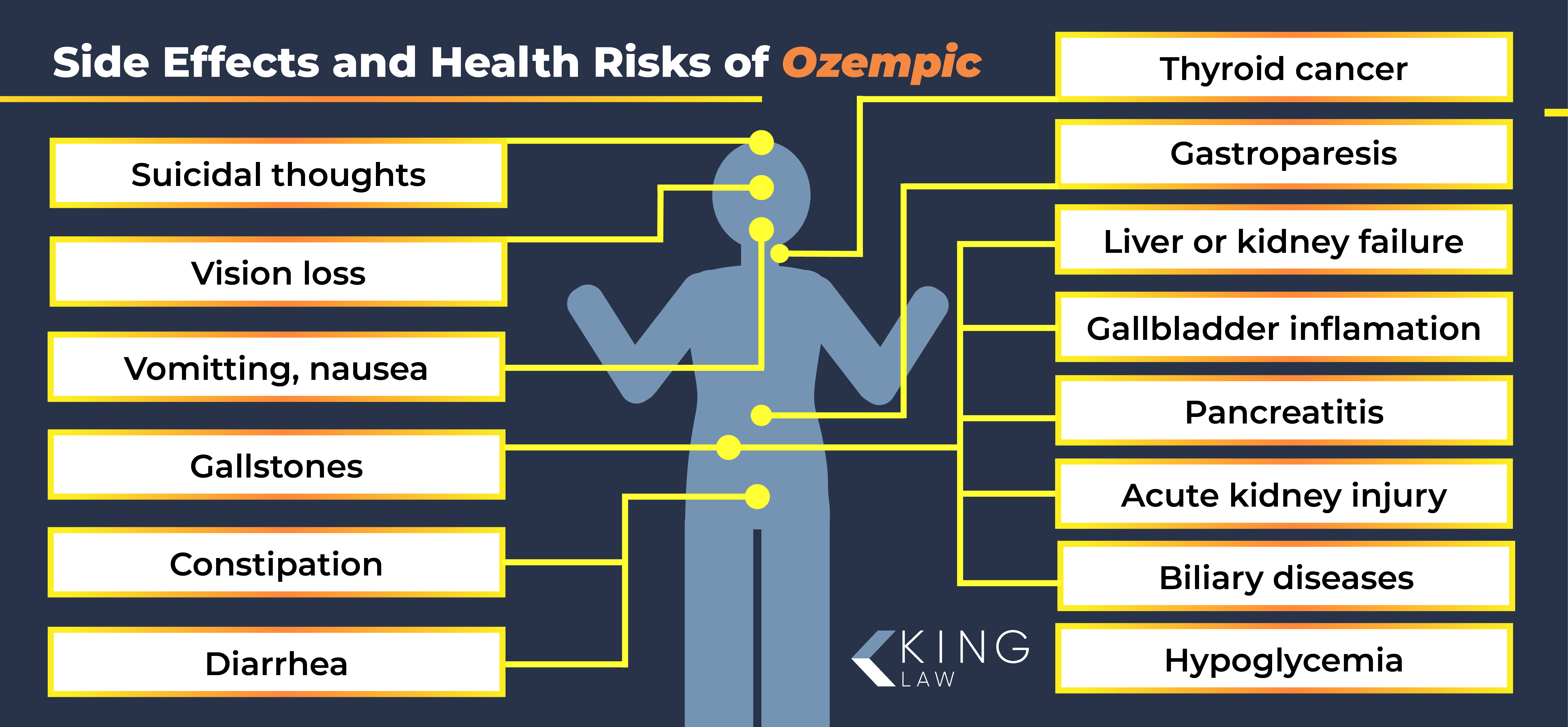
Ozempic Side Effects and Health Risks
Studies show that the use of Ozempic may lead to an increased risk of gallbladder and biliary diseases. The risk may increase the longer that a patient remains on the drug and the higher the dose.
Ozempic side effects may include:
- Acute kidney injury
- Biliary diseases
- Constipation
- Diarrhea
- Deep vein thrombosis (DVT), leading to pulmonary embolism (PE)
- Gallbladder inflammation
- Gallstones
- Gastroparesis
- Hypoglycemia
- Liver or kidney failure
- Nausea
- Pancreatitis
- Suicidal thoughts
- Thyroid cancer
- Vision changes / loss / blindness (NAION)
- Vomiting
In addition to these adverse health concerns, there is growing concern about a potential link between Ozempic and an increased risk of pancreatic cancer. The side effects of Ozempic do not necessarily go away after the drug is no longer being used. Some patients have reported continued gallbladder issues despite discontinuation of the use of the drug.
The risk of gallbladder problems is pronounced when the drug is used for long periods of time or at a higher dosage, which is typically the case when it is prescribed for weight loss. Individuals who have suffered gallbladder-related side effects after the use of Ozempic or Wegovy may be entitled to compensation through an Ozempic lawsuit due to the manufacturer’s failure to warn.
Ozempic and Vision Loss
There is a potential link between vision loss and Ozempic. In July of 2024, the medical journal JAMA Ophthalmology published a study showing a potential link between semaglutide drugs, like Ozempic, and the risk of eye strokes. The study showed an increased risk of vision loss for patients taking Ozempic and other semaglutide drugs.
The study focused on a specific injury called non-arteritic ischemic optic neuropathy (NAION). NAION occurs when the optic nerve doesn’t get enough blood flow. This lack of blood flow causes a sudden, painless loss of vision in one eye and can cause blindness.
The initial findings suggest patients who treat their diabetes with semaglutide drugs are almost three times more likely to develop NAION. The study also found patients taking semaglutide drugs for weight loss had similar outcomes.
Typically, vision loss caused by NAION is not reversible or treatable. If you or a loved one have been diagnosed with vision problems after taking Ozempic, you should contact one of our lawyers to discuss your legal options for an Ozempic vision-loss lawsuit.
Ozempic and Blood Clots/DVT
There is a possible association between taking Ozempic and developing a specific type of blood clot called a deep vein thrombosis (DVT). DVT occurs when a blood clot forms in a deep vein in the body, often in the leg. These clots can be serious or life-threatening.
The link between Ozempic and DVT was examined in a study published in the Endocrine Journal. The authors conducted a meta analysis of data from the SUSTAIN and PIONEER trials. Those two trials assessed the effectiveness of semaglutide for patients with type 2 diabetes. The authors examined data from those trials and found a 266% increased risk of DVTs for patients taking semaglutide drugs. The study stated that, “semaglutide significantly increased the risk of deep vein thrombosis.”
Ozempic and Pulmonary Embolisms
Ozempic can cause DVT, and DVT puts people at risk for developing pulmonary embolisms (PEs). Most PEs that are caused by blood clots are a result of DVT. Then DVT can lead to a PE when part of a blood clot breaks free from a deep vein and travels to the lungs. Once in the lungs, the clot can block the smaller, pulmonary arteries.
These embolisms are very dangerous. The National Institutes of Health state that PEs caused by DVT may be responsible for more than 100,000 deaths in the U.S. each year.
Ozempic Manufacturer: Novo Nordisk
Who manufactures Ozempic?
Ozempic and Wegovy are manufactured by the century-old Danish healthcare company Novo Nordisk. Since its inception in 1923, the company has focused on the treatment of diabetes through the extraction of insulin.
Prior to the development of Ozempic, the drug manufacturer sold Victoza, a once-daily GLP-1 injectable. The pharmaceutical company came under scrutiny for promoting Victoza for off-label uses, eventually agreeing to pay a $1.1 million whistleblower settlement. The company also agreed to pay $58 million to resolve lawsuits related to their failure to comply with an FDA-mandated risk program.
Novo Nordisk is currently facing litigation related to its failure to warn consumers about the potential risks associated with the use of Ozempic and Wegovy. New lawsuits allege that the manufacturer failed to put on the warning label the potential issues that can occur while using the drugs.

Eligibility Criteria for Filing an Ozempic Lawsuit
Individuals who have received a diagnosis for an adverse health condition after taking Ozempic, Rybelsus, or Wegovy GLP-1 receptor agonist treatments may be entitled to take legal action.
Eligibility criteria for filing an Ozempic lawsuit include:
- Proof that you received Ozempic, Rybelsus, or Wegovy treatments
- A diagnosis of an adverse health condition such as gastroparesis, stomach paralysis, ileus, bile duct cancer, Lou Gehrig’s Disease (ALS), gallbladder cancer, sinus cancer, vision loss, deep vein thrombosis, pulmonary embolism, or gastric intestinal obstruction during treatment. Our lawyers are also looking at the possibility of cases involving aspiration from blood clots.
- An emergency room visit, hospitalization, or visit with a gastroenterologist or ophthalmologist, related to the condition.
It is critical to consult with an Ozempic lawyer to determine if you meet the criteria requirements and for state-specific statutes of limitations that may affect your case.

How to File an Ozempic Lawsuit
If you were diagnosed with Gastroparesis, Stomach Paralysis, Ileus, or Gastric Intestinal Obstruction during treatment or within 30 days of using the drug. related to the use of Ozempic or Wegovy, you might be entitled to compensation for your injuries. An Ozempic lawyer can help you understand your rights and determine whether you meet the criteria necessary to take legal action.
An Ozempic lawyer can help you by:
- Offering a free case review to verify lawsuit eligibility;
- Collecting evidence to support your claim, including medical records and witness testimonies;
- Determining state-specific deadlines and statute of limitations;
- Handling settlement negotiations; and
- Setting the case for trial if a favorable settlement cannot be reached.
It is essential that you consult with a lawyer as early in the process as possible. During your meeting, you need to supply the attorney with all of your health information and supporting documentation related to your claim.
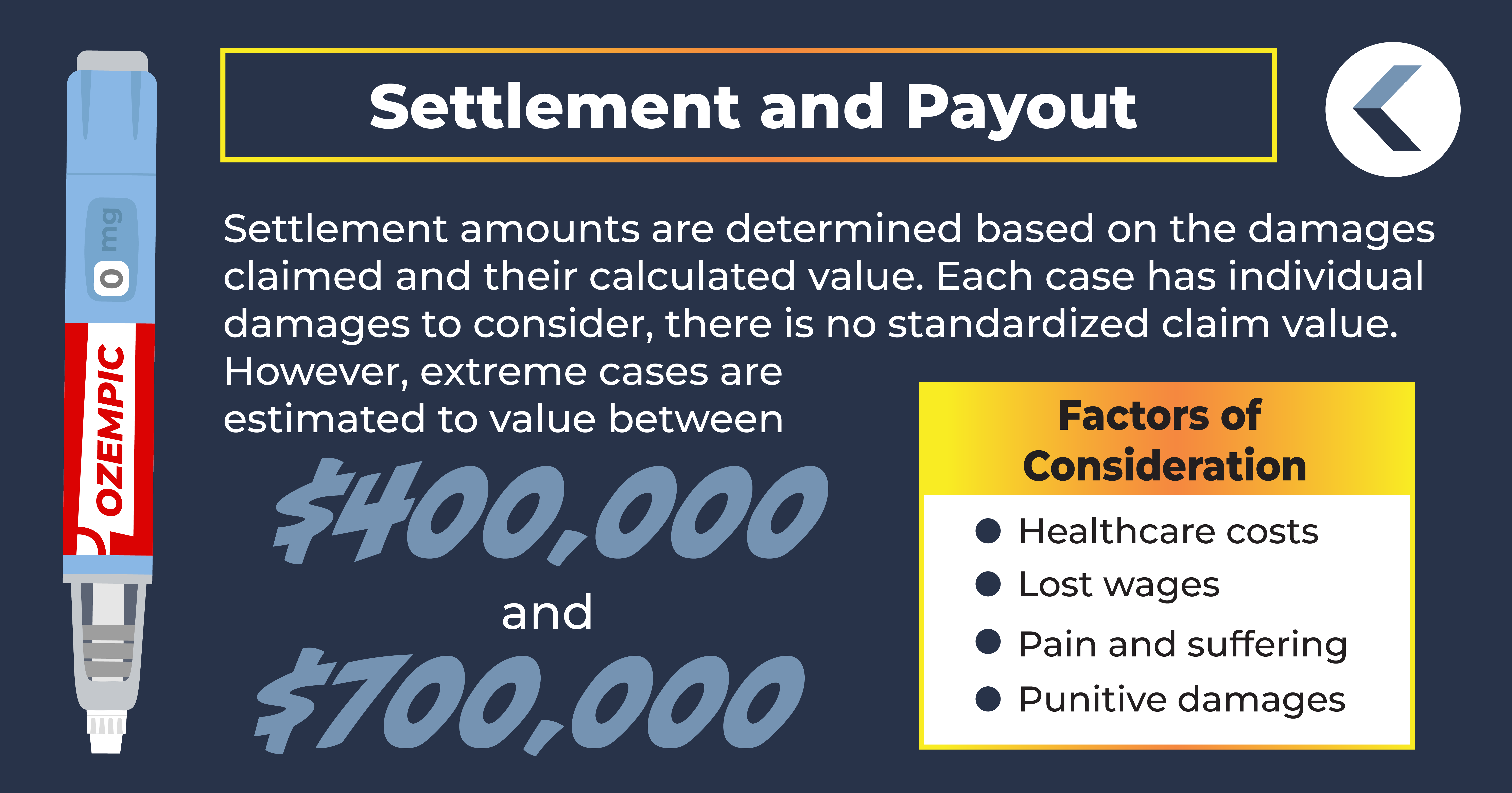
Ozempic Settlement Amounts
In Ozempic lawsuits, the primary determining factor in a potential settlement amount is the damages claimed and their calculated value. Since cases vary widely based on their individual damages, there is no standardized claim value. The estimated settlement value for cases involving gallbladder removal or wrongful death, however, is approximately $400,000 to $700,000.
Damages in an Ozempic lawsuit may include compensation for losses related to:
- Healthcare costs
- Lost wages
- Pain and suffering
- Punitive damages
An attorney can help to maximize your compensation by filing a claim based on the full range of available damages. They can also provide guidance on what documentation is needed to demonstrate the value of your losses and negotiate to ensure a fair and full settlement is offered.
Contact an Ozempic Lawyer Today
At King Law, our Ozempic lawyers provide dedicated representation for individuals who have suffered adverse health conditions as a result of taking Ozempic, Wegovy, or other GLP-1 receptor agonists. We have years of experience advocating on behalf of those injured by dangerous drugs.
Our Ozempic attorneys will work tirelessly to ensure you receive the best possible outcome in your case. Contact our office today to schedule a free, no-obligation consultation. We proudly represent clients nationwide.


 Understanding the Ozempic Lawsuits: A Comprehensive Guide
Understanding the Ozempic Lawsuits: A Comprehensive Guide