
Mounjaro Lawsuit Overview
Mounjaro is the brand name for a drug called tirzepatide, manufactured by pharmaceutical giant Eli Lilly and Company. The drug is used for glycemic control in Type 2 diabetes, but clinical trials showed significant weight loss benefits for people with an elevated body mass index (BMI). It is widely considered a successor to Ozempic, which took the market by storm.
Almost immediately, there were reports of severe side effects, including gastroparesis and gastroenteritis. Despite the reported adverse events, there have been no recalls issued for the drug. Lawsuits now allege that the manufacturer of Mounjaro knew the potential for severe side effects and failed to warn consumers.
Our firm is currently investigating Mounjaro lawsuits involving a diagnosis of:
- Gastroparesis
- Stomach Paralysis
- Gastrointestinal Obstruction
- Illeus
- Daily vomiting lasting at least 3 weeks
- Bile Duct Cancer
- Lou Gehrig’s Disease (ALS)
- Gallbladder Cancer
- Sinus Cancer
Individuals who have suffered adverse health conditions after using Mounjaro are strongly encouraged to contact King Law to schedule a free, no-obligation consultation.
Mounjaro Lawsuit Update – July 2024
June 17, 2024 – Mounjaro Lawsuit Advances with First Status Conference Under New Judge Karen Marston
June 9, 2024 – Judge Karen Spence Marston to Preside Over Mounjaro Lawsuit
May 20, 2024 – Judge Change in Mounjaro Lawsuit Following Judge Pratter’s Death
May 2, 2024 – Mounjaro Lawsuit “Science Day” Scheduled for June 14, 2024
April 25, 2024 – Mounjaro Lawsuit Initiated with Plaintiffs’ First Statement of the Case
April 20, 2024: Mounjaro Lawsuits Still in Early Stages
To date, the lawsuits against the maker of Mounjaro are still in the early stages of litigation. No settlements or trials are scheduled yet. However, litigation is expected to grow significantly due to life-altering side effects.
February 29, 2024: Mounjaro Lawsuits Consolidated into Multidistrict Litigation in Pennsylvania
With the number of lawsuits against the manufacturer of Mounjaro increasing, federal cases are consolidated into multidistrict litigation (MDL). Pre-trial proceedings will be heard in the Eastern District of Pennsylvania.
November 30, 2023: FDA Approves Zepbound for Chronic Weight Management in Adults
The FDA approves Zepbound as a treatment for chronic weight management in adults with obesity. Zepbound has the same active ingredient as Mounjaro, tirzepatide, and is manufactured by Eli Lilly.
August 28, 2023: Lawsuit Filed Against Makers of Mounjaro and Ozempic Over Side Effects Warning
CNN reports that a lawsuit has been filed against the makers of Mounjaro and Ozempic, alleging that they failed to warn consumers about the potential for severe side effects when using their drugs.
May 26, 2022: Mounjaro Approved for Treatment of Type 2 Diabetes in Adults
The U.S. Food and Drug Administration (FDA) approves Eli Lilly’s Mounjaro™ (tirzepatide) for treatment in adults with Type 2 diabetes.
On this page:
What Is Mounjaro and How Does It Work?
Mounjaro vs. Ozempic: The Similarities and Differences
Mounjaro Side Effects and Symptoms
Long-Term Side Effects of Mounjaro
Mounjaro Usage Linked to Stomach Paralysis
Mounjaro Manufacturer: Eli Lilly and Company
Mounjaro FDA-Approval and Its Black Box Warning
Mounjaro Active and Inactive Ingredients
Eligibility Criteria for Filing a Mounjaro Lawsuit
Statute of Limitations for a Mounjaro Lawsuit
How to File a Mounjaro Lawsuit
What Is Mounjaro and How Does It Work?
Mounjaro is a weekly injectable approved for the treatment of Type 2 diabetes in adults. It is the brand-name medication for tirzepatide, manufactured by Eli Lilly and Company. Mounjaro’s active ingredient works as a dual agonist for the GLP-1 (glucagon-like peptide-1) receptor and the GIP (glucose-dependent insulinotropic polypeptide) receptor. This dual-action quality makes it unique to its predecessors. There is no generic version of the drug available on the market.
The drug was initially approved by the U.S. Food and Drug Administration on May 13, 2022, to manage blood sugar in adults with Type 2 diabetes. It is often prescribed alongside the recommendation for exercise and a balanced diet. The medication works by increasing insulin release and reducing sugar production in the liver. Ultimately, this process slows food emptying from the stomach, enhancing satiety. It is not approved for the treatment of Type 1 diabetes and is not to be started by individuals with current or past pancreatitis.
In November 2023, a version of the drug was approved for chronic weight management in adults with obesity or who are overweight with at least one weight-related condition. The drug is reportedly effective for weight loss because it mimics hormones that regulate appetite and blood sugar (GLP-1 and GIP). The suppression of appetite and alteration of stomach emptying weights has led to significant weight loss for some patients.

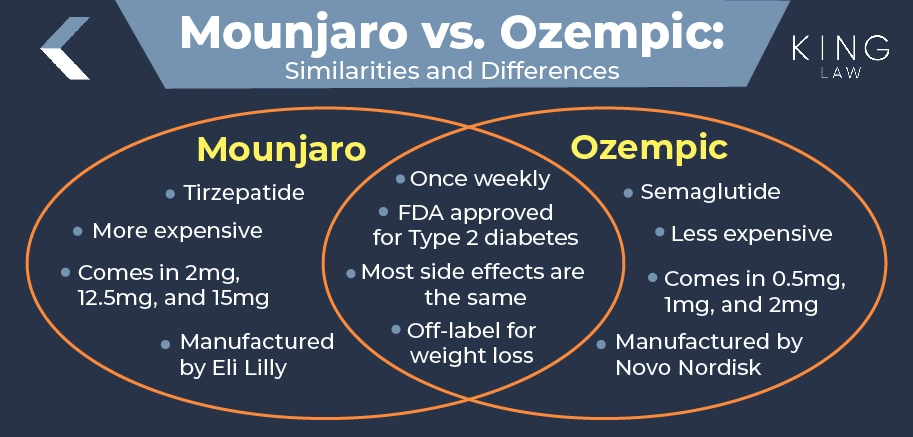
Mounjaro vs. Ozempic: The Similarities and Differences
While Mounjaro and Ozempic treat the same conditions, the underlying drug and their mechanisms are substantially different. Both drugs are FDA-approved for management of Type 2 diabetes. Ozempic, however, is also approved for reducing cardiovascular risks in people with Type 2 diabetes who have established cardiovascular disease.
Mounjaro is known for being more expensive than its counterpart; however, studies show that it is more effective in controlling blood sugar and aiding in weight loss. The drug’s mechanism of action is also unique. The active ingredient in Ozempic is semaglutide, a GLP-1 receptor agonist. The underlying drug in Mounjaro is a dual GIP and GLP-1 receptor agonist known as tirzepatide. It is the first of its kind on the market.
Both drugs are administered in weekly subcutaneous injections. The dosages differ substantially. Ozempic is available in 0.5 mg, 1 mg, and 2 mg dosages, while Mounjaro is available in 2 mg, 12.5 mg, and 15 mg dosages. Each drug may be prescribed off-label for weight loss and is effective in controlling blood sugar levels in patients with Type 2 diabetes. Unfortunately, both have also been linked to severe side effects. In some instances, Mounjaro may have additional side effects because of its dual-action mechanism.
Mounjaro Side Effects and Symptoms
The side effects of Mounjaro range from mild to severe, with some lasting long-term. One of the most concerning reported events after taking Mounjaro is serious gastrointestinal issues such as gastroparesis. These issues are commonly reported by individuals who were prescribed the drug for off-label obesity treatment.
Mounjaro side effects and symptoms:
- Gastroparesis (stomach paralysis)
- Ileus or intestinal blockage
- Disruption of normal digestion
- Gastroenteritis
- Cramps
- Diarrhea
- Severe or excessive vomiting
- Severe dehydration
- Pancreatitis
- Renal (kidney) complications
- Abdominal pain
- Gallbladder issues
- Delayed gastric emptying
- Acute kidney injury
- Risk of thyroid cancer
- Abdominal swelling
- Severe allergic reaction
Lawsuits that have been filed against Eli Lilly, the manufacturer of Mounjaro, allege that the maker did not provide adequate warnings about the potential for serious gastrointestinal effects after using the drug.
Long-Term Side Effects of Mounjaro
Long-term side effects of Mounjaro may include serious conditions such as prolonged vomiting and severe digestive issues such as stomach paralysis. Individuals with these conditions are strongly encouraged to seek legal counsel as soon as possible.
Potential long-term side effects of Mounjaro may include:
- Prolonged vomiting (Patient reports indicate it may last for weeks)
- Severe digestive issues
- Stomach paralysis (gastroparesis)
- Pancreatitis
Seeking prompt medical attention for any side effects you experience can help ensure you receive the care and treatment you need. It is essential to retain any medical records related to your condition. Evidence often plays a critical role in Mounjaro-related lawsuits.

Mounjaro Usage Linked to Stomach Paralysis
One of the most concerning reported side effects of Mounjaro usage is its link to stomach paralysis or gastroparesis. Gastroparesis is a condition where the movement of the stomach muscles is disrupted. The disruption affects how food is propelled through the digestive system, which leads to slowed or halted stomach emptying. Patients with Type 2 diabetes have an increased risk of gastroparesis because of the potential for vagus nerve damage.
Recent studies highlight the increased risk for gastrointestinal issues when taking a GLP-1 receptor agonist like Mounjaro. Higher doses correlate with a more significant risk of nausea, vomiting, and gastroparesis. Because Mounjaro is prescribed at a higher dose compared to similar treatments, these side effects may be more severe.
Research also suggests a link between GLP-1 medications and adverse psychiatric events, although more studies are needed to detail the connection. Allegations against the manufacturer of Mounjaro include that the company failed to warn individuals about the risk of severe side effects, specifically for those predisposed to gastroparesis.
Mounjaro Manufacturer: Eli Lilly and Company
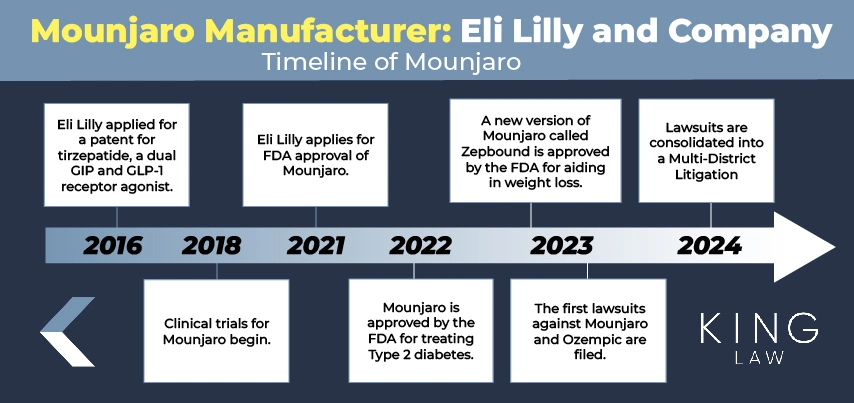
In early 2016, Mounjaro manufacturer Eli Lilly and Company applied for a patent for tirzepatide. The patent was published later that year. The pharmaceutical company then applied for FDA approval with a priority review voucher in October 2021 and received approval for the drug on May 13, 2022, for the treatment of Type 2 diabetes in adults.
The medication’s international nonproprietary name (INN) is Tirzepatide; however, it is sold under the brand names Mounjaro and Zepbound. In November 2023, the FDA approved Zepbound for weight management in adults with obesity.
Within a year of approval, the FDA began receiving reports of adverse health events from patients using the drug. These reports have also resulted in legal actions against the manufacturer. Allegations include that Eli Lilly failed to adequately warn users about the risk of adverse medical events, specifically gastroparesis. These allegations have led to questions about the drug’s safety and efficacy.
Mounjaro FDA-Approval and Its Black Box Warning
After applying for a patent for the drug tirzepatide in early 2016 and receiving publication later that year, Eli Lilly applied for approval with a priority review voucher in October 2021. The company received FDA approval for Mounjaro to treat Type 2 diabetes in adults in May 2022 following a priority review. On November 8, 2023, the FDA approved Zepbound for use in adults with obesity or those who are overweight with at least one weight-related condition.
The black box warning notes that GLP-1s such as Mounjaro are contraindicated for individuals with a history or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2.
Mounjaro Active and Inactive Ingredients
Mounjaro is a weekly injectable and the active ingredient is tirzepatide. It is available in a pack containing four single-dose prefilled pens or in individual single-dose vials with a cardboard outer carton.
Inactive ingredients in Mounjaro:
- Sodium chloride
- Sodium phosphate dibasic heptahydrate
- Water for injection
The inactive ingredients are generally used for stabilization and pH adjustment. The pH may be adjusted with a hydrochloric acid solution and/or sodium hydroxide solution.
Eligibility Criteria for Filing a Mounjaro Lawsuit
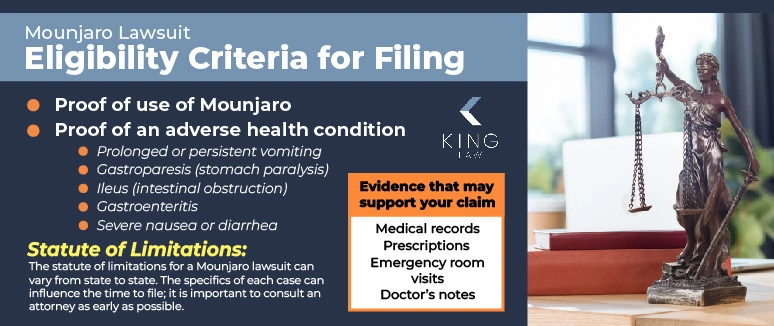
In order to file a Mounjaro lawsuit, you must meet certain eligibility requirements. The best way to determine whether you meet the necessary criteria for filing a claim is by consulting with an experienced attorney.
Eligibility criteria for filing a claim:
- Proof of use of Mounjaro.
- Proof of adverse health condition (prolonged or persistent vomiting, gastroparesis, ileus, gastroenteritis, nausea, bile duct cancer, Lou Gehrig’s Disease (ALS), gallbladder cancer, sinus cancer, or diarrhea).
It is important to note that not all individuals who suffered a side effect after using Mounjaro will qualify for a lawsuit. An attorney can review your case to help determine whether you qualify during a consultation. If you qualify, you may be able to seek compensation for damages, including your medical expenses and lost wages.
Evidence to Collect for a Mounjaro Lawsuit:
In order to prove your claim, you may need to provide evidence. Working with an attorney can help ensure you collect the evidence you need to strengthen your case.
Evidence that may substantiate your claim:
- Medical records
- Prescriptions
- Emergency room visits
- Doctor’s notes
Statute of Limitations for a Mounjaro Lawsuit
The statute of limitations for a Mounjaro lawsuit can vary by state law. It is imperative to consult with an attorney as early in the process as possible to ensure you meet all legal requirements, including the statute of limitations. There may be several factors that can influence or extend the amount of time you have to file a claim including the state where the case is filed and the specifics of your diagnosis.
How to File a Mounjaro Lawsuit
There are multiple steps that you need to take to file a Mounjaro lawsuit. Working with an attorney can help you understand the process and ensure you receive the best possible outcome in the case.
Steps for filing a Mounjaro lawsuit:
- Initial consultation: As soon as possible, you should contact a lawyer for a free case review to determine your eligibility.
- Evidence gathering: Mounjaro lawsuits generally require evidence that supports the claim. Evidence may include medical records, testimonies, and documentation. Proof of damages is also essential which can be supported by evidence of your medical expenses, diagnosis, and purchase of Mounjaro.
- Filing the lawsuit: Your attorney and legal team will handle all paperwork related to your case and will make sure your claim is filed within the state-specific deadlines.
- Negotiation and court proceedings: Your attorney will work with the defendant(s) to negotiate a favorable settlement or set the matter for trial if a settlement cannot be reached.
Mounjaro Settlements and Payout Amounts
The anticipated settlement range for serious injuries in a Monjauro lawsuit is between $400,000 and $700,000; however, settlement values may vary depending on the case. There is no standardized settlement amount. The value of the settlement will depend on the individual damages in the case and may be based on factors like the extent of losses and injuries.
An experienced attorney can help maximize compensation. Without the help of an attorney, you may not be aware of the full range of damages or be able to secure the documentation necessary to prove your case.
Contact a Mounjaro Lawyer
Did you suffer gastroparesis or another adverse health condition after taking Mounjaro? You may be eligible for compensation. At King Law, we have extensive experience helping individuals who have had serious side effects after taking Mounjaro and other weight-loss drugs. Contact our office today to start the process of determining eligibility and filing a lawsuit against the makers of Mounjaro.
Frequently Asked Questions (FAQs)
Find answers to any major questions you have regarding eligibility in the Mounjaro lawsuits below.

