
Saxenda is an injectable prescription drug used to aid weight loss in adults and teens who suffer from obesity, high BMI, or other weight-related medical problems. The medication has increased significantly in popularity, with demand being so great that it has impacted the availability of the drug.
The medication, however, has serious side effects. Current lawsuits allege the drug may cause gastroparesis and other severe health risks. As of February 2024, many Saxenda lawsuits have been consolidated into multidistrict litigation (MDL 3094). There are over 100 pending cases with litigation ongoing. The number of lawsuits is expected to grow significantly based on the widespread use of these drugs.
Saxenda Lawsuit Overview
People are filing Saxenda lawsuits after experiencing a range of severe side effects, including stomach and bowel injuries, vision loss, and blood clots. Saxenda, a prescription-only weight-loss medication, is manufactured by Novo Nordisk. Its popularity has been increased substantially by social media campaigns and celebrity endorsements. Originally marketed for diabetes management, the drug is now used for weight loss in teens and adults.
As with other Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RAS), Saxenda has been linked to serious health conditions, including stomach paralysis or gastroparesis. In some cases, the side effects are so severe that they require hospitalization. Additional accusations against the manufacturer include that the medication may cause pancreatic cancer.
At King Law, our attorneys are reviewing claims linked to Saxenda use. Recent studies and case reports indicate that the weight loss drug may cause serious health risks, including gastroparesis.
Our firm is currently investigating Saxenda lawsuits involving a diagnosis of:
- Gastroparesis/stomach paralysis
- Gastrointestinal obstruction/ileus
- Blindness and/or vision problems (NAION)
- Daily vomiting lasting at least 3 weeks
- Blood clots/deep vein thrombosis (DVT) or pulmonary embolism (PE)
Affected individuals are encouraged to contact our office to discuss their legal options. Consultations are provided free of charge and without obligation to retain our services.

Saxenda Lawsuit – 2025 Update
November 7, 2024: Judge in Saxenda MDL Issues Order on Expert Report Timelines
October 4, 2024: Wave of Lawsuits Against Novo Nordisk Intensifies as Side Effects of Saxenda Cause Harm
October 3, 2024: Saxenda Trial Shows Temporary Weight Loss in Children, Raises Safety Concerns
August 2024: A comprehensive analysis examining how semaglutide drugs may lead to blood clots, deep vein thrombosis (DVT), and pulmonary embolisms is gaining new traction. The analysis, published in the Endocrine Journal, saw a 266% increase of DVT in patients taking semaglutide drugs, such as Ozempic and Wegovy. More research will be conducted to see if other GLP-1 agonist drugs, like Saxenda, cause blood clots and DVT.
July 2024: A recent study published in JAMA’s Ophthalmology examined a possible tie between taking semaglutide drugs and suffering vision loss. The study found people taking these drugs had a four times greater likelihood of having an eye stroke. The strokes are called anterior ischemic optic neuropathy (NAION) and can cause vision loss or blindness. Medical professionals will be paying close attention to see if other GLP-1 agonist drugs, like Saxenda, may cause NAION.
June 2024: The MDL is reassigned to Judge Karen Spencer Marston after the unexpected death of the previous presiding judge. There are currently over 100 cases pending in the MDL with the number expected to increase rapidly in the coming months.
February 2024: Lawsuits against Saxenda manufacturer Novo Nordisk are consolidated into multidistrict litigation out of the Eastern District of Pennsylvania (MDL 3094 In Re: Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAS) Products Liability Litigation).
About the Saxenda Lawsuit:
Saxenda Side Effects and Health Risks
Long-Term Side Effects of Saxenda
Saxenda FDA Approval and Its Black Box Warning
Saxenda Manufacturer: Novo Nordisk
Saxenda Active and Inactive Ingredients
Saxenda and Gastroparesis: Studies Link Liraglutide to Stomach Paralysis
Eligibility Criteria for Filing a Saxenda Lawsuit
Statute of Limitations for a Saxenda Lawsuit
Saxenda Settlement and Payout Amounts
What Is Saxenda?
Saxenda is a daily injectable prescription medication manufactured by Novo Nordisk. It has the same active ingredient in Victoza, liraglutide, a GLP-1 receptor agonist similar to semaglutide. The mechanism of action of the drug works by mimicking the GLP-1 hormone, targeting the areas of the brain that regulate appetite and food intake.
The drug, following a favorable vote from the Endocrinologic and Metabolic Drugs Advisory Committee, was approved in December 2014 by the U.S. Food and Drug Administration (FDA) for chronic weight management in adults with obesity (BMI of 30 or higher) or overweight (BMI of 27 or higher) and at least one weight-related condition, such as type 2 diabetes, high blood pressure, or high cholesterol. Saxenda was launched to market in the United States on April 22, 2015.
In December 2020, the FDA issued approval for the drug to be used in adolescents as young as 12 years old with a body weight above 132 pounds (60 kg) with obesity. In both instances, the drug was to be used as part of a broader weight management program in conjunction with a reduced-calorie diet and increased physical activity.
How Does Saxenda Work?
Saxenda’s mechanism of action is similar to that of other popular weight loss medications that have recently become more popular. It is a glucagon-like peptide-1 (GLP-1) receptor antagonist, meaning it mimics the GLP-1 hormone in the brain. It is chemically 97% similar to this hormone, making it extremely effective. In essence, it works in the hypothalamus of the brain, interacting with specific neurons that regulate appetite and food intake.
By mimicking the GLP-1 hormone, it helps to lower hunger stimulation, ultimately leading to a reduction in food intake. Additionally, it helps the stomach to digest food more slowly, causing a prolonged feeling of fullness. The medication comes in a liquid solution inside of a prefilled pen. The drug is injected once daily under the skin on the stomach or thigh. Currently, there is no generic available.
Common side effects associated with using Saxenda include nausea, vomiting, diarrhea, constipation, and low blood sugar (hypoglycemia). However, patients report severe health conditions related to taking the medication, including stomach paralysis (gastroparesis) and potential pancreatic cancer. The drug continues to be reviewed for its safety and effectiveness as a result of these alleged severe side effects and potential legal issues.
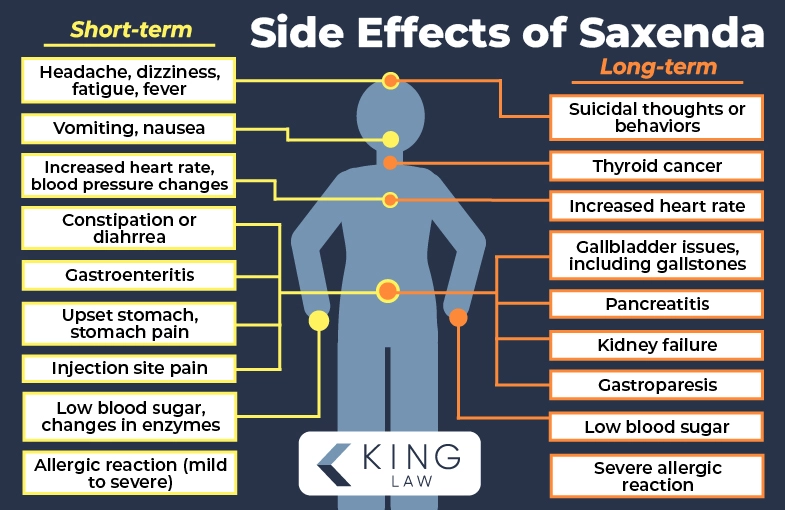
Saxenda Side Effects and Health Risks
Saxenda is associated with a number of side effects and potential health risks. Similar to other GLP-1 receptor agonists, the active ingredient in the medication, liraglutide, has been linked with several gastrointestinal effects. Due to its mechanism of action regulating appetite and insulin secretion, it may impact multiple systems within the body.
Side effects associated with Saxenda:
- Headache
- Dizziness
- Fatigue
- Fever (common in teens)
- Nausea
- Vomiting
- Diarrhea
- Constipation
- Gastroenteritis (inflammation of the stomach and intestines)
- Upset stomach (dyspepsia)
- Stomach pain
- Low blood sugar (hypoglycemia)
- Change in enzyme (lipase) levels in your blood
- Mild allergic reaction (rash, itching)
- Severe allergic reaction (rare cases)
- Increased heart rate (heart palpitations, chest pain)
- Changes in blood pressure and other cardiovascular issues
- Redness, pain, or swelling at the injection site
Lawsuits allege that Novo Nordisk, the manufacturer of Saxenda, failed to warn of adverse side effects related to the long-term use of the drug, including the potential for gastroparesis. Research indicates that the drug slows down gastric emptying and may adversely affect digestive system functions.
Long-Term Side Effects of Saxenda
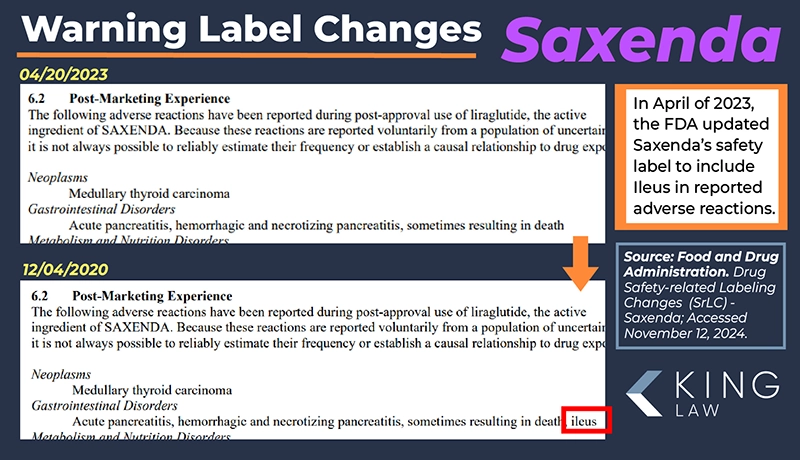
Long-term side effects associated with Saxenda include gastroparesis, ileus, pancreatitis, and potential thyroid tumors. Individuals taking Saxenda are encouraged to seek regular checkups and report adverse reactions to their medical provider as soon as possible.
Long-term side effects linked to Saxenda:
- Gastroparesis (stomach paralysis)
- Ileus (intestinal blockages)
- Gallbladder problems, including gallstones (symptoms include severe abdominal pain, diarrhea, pale-colored stool, fever, nausea, vomiting, jaundice)
- Increased heart rate (heart palpitations, chest pain)
- Pancreatitis (sudden upper abdominal pain, persistent severe burning pain in the abdomen, vomiting)
- Suicidal thoughts or behavior (new or worsening depression, thoughts about harming yourself or ending your life, changes in mood or behavior)
- Kidney failure
- Low blood sugar
- Risk of thyroid cancer
- Severe allergic reaction
Saxenda and Vision Loss
A recent study in JAMA’s Ophthalmology found semaglutide drugs could cause a specific sort of eye condition called NAION. Although Saxenda is not a semaglutide drug, it belongs to the same class of drugs as semaglutide. Both semaglutide and liraglutide, the active ingredient in Saxenda, are GLP-1 agonist receptor drugs.
This condition is called nonarteritic anterior ischemic optic neuropathy (NAION), which can cause permanent vision loss or blindness. More studies will be needed to discover if all GLP-1 drugs carry an increased risk of NAION and the associated vision loss. Current information suggests that people who take semaglutide drugs are three times more likely to develop NAION than people who do not.
Saxenda and Blood Clots
Some GLP-1 drugs have been shown to increase the risk of blood clots. A study published in the Endocrine Journal studied data from more than 12,000 patients who used semaglutide to treat their diabetes. The data pointed to a 266% increased risk of a certain type of blood clot for people taking semaglutide drugs. The blood clots are called deep vein thrombosis (DVT), and they can lead to severe complications such as pulmonary embolisms.
Although the active ingredient in Saxenda (liraglutide) is different than semaglutide, both drugs belong to the same class—GLP-1 agonist receptors. More research will be needed to determine if other GLP-1, like Saxenda, can increase the risk of blood clots.
Saxenda FDA Approval and Its Black Box Warning
Saxenda received FDA approval on December 23, 2014, for weight loss management in adults with obesity or who are overweight (BMI of 27 kg/m² or higher) with at least one weight-related medical condition. Weight-related medical conditions include but are not limited to high blood pressure, type 2 diabetes, and high cholesterol. Approval was based on clinical trials that involved over 5,000 overweight or obese individuals. In December 2020, approval was granted for pediatric patients ages 12 and older who are obese and weigh 132 pounds or more.
It is recommended that patients using the drug be evaluated after 16 weeks and that the drug is discontinued if less than 4% of the baseline body weight is lost. The medication carries a black box warning, the FDA’s highest level of warning. The black box warning highlights the risk of thyroid cancer and acute pancreatitis. The risk of thyroid cancer is based on animal studies and has not been confirmed in humans. It is, however, contraindicated for individuals with a personal or family history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia syndrome type 2 (MEN 2).
Other potentially serious adverse events linked to the use of the drug include acute gallstone disease and thyroid neoplasm. Due to the aforementioned warnings, it is strongly recommended that patients using Suboxone undergo continuous evaluation for thyroid cancer and pancreatitis symptoms.
Saxenda Manufacturer: Novo Nordisk
Novo Nordisk, the manufacturer of Saxenda (liraglutide), is a pharmaceutical company based in Denmark. In July 2023, the company experienced a rapid increase in demand for the drug. As a result of the increase in demand, the manufacturer announced that it anticipated challenges in filling prescriptions for the drug throughout 2023 and beyond. The shortages have caused the company to expand and build new production facilities to meet the demand for Saxenda and other GLP-1 products.
Saxenda launched to market in the U.S. in April 2015. It is alleged that the company planned to use 500 of its nearly 3,000-person sales force to promote the drug. It is estimated that the manufacturer spent $1 billion over a decade in research and marketing.
Despite these efforts, the company has faced numerous controversies and legal issues. In 2010, it was alleged that Novo Nordisk breached the Association of the British Pharmaceutical Industry (ABPI) code by failing to provide information about the drug’s side effects and promoting the medication before it received market authorization. The company was recently suspended from membership.
In 2012, Public Citizen, a nonprofit consumer advocacy organization, petitioned the FDA to remove liraglutide (the active ingredient in Victoza and Saxenda) from the market due to the risks of thyroid cancer and pancreatitis.
In September 2017, the company agreed to pay $58.65 million for failing to comply with the FDA-mandated Risk Evaluation and Mitigation Strategy (REMS) related to illegal marketing and promotion of the Type 2 diabetes medication Victoza (liraglutide) for off-label uses. The company also paid $1.45 million to California and Illinois to settle fraud cases against private commercial health insurers.
Saxenda Active and Inactive Ingredients
The active ingredient in Saxenda is liraglutide. Liraglutide is a GLP-1 receptor agonist that regulates appetite, slows gastric emptying, and enhances insulin secretion in response to meals, aiding in weight management and blood glucose control. The medication contains 6 mg per 1 mL of liraglutide.
Inactive ingredients in Saxenda:
- Disodium Phosphate Dihydrate (1.42 mg per 1 mL): Acts as a buffering agent to maintain the pH and stability of the solution.
- Propylene Glycol (14 mg per 1 mL): Solvent and stabilizer that helps dissolve the active ingredient and ensures consistency.
- Phenol (5.5 mg per 1 mL): Preservative that prevents microbial growth and contamination.
- Water for Injection: Solvent that carries all the ingredients, allowing the medication to be administered via injection.
The medication is taken once daily at any time of day without regard to meals. It is injected subcutaneously in the abdomen, thigh, or upper arm. The injection site can be changed without adjustment to the dose but should not be administered intravenously or intramuscularly.
Saxenda dosage and administration:
- Week 1: 0.6 mg
- Week 2: 1.2 mg
- Week 3: 1.8 mg
- Week 4: 2.4 mg
- Week 5 and onward: 3.0 mg
Before opening, Saxenda should be stored in a refrigerator (2°C to 8°C) and should not be frozen. During use, the drug may be stored at room temperature (below 30°C) or in a refrigerator for up to one month. The prescription should be stored without the needle attached. Each pre-filled pen contains 18 mg of liraglutide per 3 mL, allowing for multiple doses of the medication.
Saxenda and Gastroparesis: Studies Link Liraglutide to Stomach Paralysis
Research, including recent studies, has linked liraglutide to serious gastrointestinal issues, including gastroparesis or paralysis of the stomach. Gastroparesis is a condition in which the stomach muscles do not function properly, causing food to move slowly or unpredictably into the small intestine. Common symptoms include nausea, vomiting, upper abdominal pain, bloating, and feeling full. These problems may affect a person’s quality of life, leading to missed work and increased hospital visits.
Studies indicating risks associated with Saxenda:
- Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss (JAMA)
- Medication-Induced Gastroparesis: A Case Report (Journal of Investigative Medicine – High Impact Case Reports)
- Liraglutide-induced Acute Gastroparesis (Cureus): A case study of a 52-year-old man with well-controlled diabetes who experienced acute gastroparesis after starting liraglutide. Symptoms improved with gastric suctioning and discontinuation of liraglutide.
Evidence suggests GLP-1 receptor agonists inhibit gastric emptying through parasympathetic (vagal) afferent-mediated central mechanisms. Studies show liraglutide delays gastric emptying at five and 16 weeks of treatment. Patients and healthcare providers should be aware of the potential risk of gastroparesis with Saxenda. Monitoring and appropriate management are crucial for those exhibiting symptoms after starting the medication.
Eligibility Criteria for Filing a Saxenda Lawsuit
In order to file a lawsuit based on Saxenda-related gastrointestinal issues, you must meet certain eligibility requirements. These requirements include proof of use of the medication and the development of a qualifying condition. It is strongly recommended that Saxenda patients experiencing adverse health conditions seek legal counsel as soon as possible.
Saxenda lawsuit eligibility criteria:
- Proof of Use: Individuals must be actively using Saxenda for weight loss purposes.
- Health complications: Must be able to prove medical intervention (hospitalization, ER visits, or consultations with a gastroenterologist) for the development of ileus, gastroparesis, gastrointestinal blockage, stomach paralysis, or related conditions after taking Saxenda.
- Medical Documentation: Evidence from a doctor or gastroenterologist linking the condition to Saxenda use.
If you believe that you meet these eligibility requirements, it is recommended that you contact a lawyer for a free case evaluation. An attorney can help determine the viability and value of your case. It is important to act fast, as all cases are subject to a state-specific statute of limitations. If the statute of limitations has expired, legal action cannot be taken.
Evidence to Collect Before Filing Your Lawsuit:
- Medical records
- Proof of prescriptions
- Bills and documentation from ER visits or hospitalizations
- Doctor’s notes
- Expert testimony

Statute of Limitations for a Saxenda Lawsuit
The statute of limitations for a Saxenda lawsuit varies by state. In most cases, individuals have between one and three years from the date of diagnosis to file a claim. However, legal deadlines may be extended based on the date of discovery. It is important to consult with an attorney to ensure that your case is filed within the legal timeframe established by state law.
How to File a Saxenda Lawsuit
There are certain steps you should follow to file a Saxenda lawsuit. These steps can help ensure that your claim is filed timely and in accordance with all legal requirements.
A step-by-step guide to filing a Saxenda lawsuit:
- Consult with an attorney: If you suffered adverse health conditions after taking Saxenda, you should contact a lawyer to obtain a free case review. During your initial consultation, the attorney will determine whether you may be eligible to take legal action.
- Collect evidence: Prior to filing, you will want to gather evidence, including your medical records, expert testimony, and any documents related to your case, such as proof of medical expenses, diagnosis, and purchase of Saxenda.
- Filing the lawsuit: Once you have the evidence you need, your attorney will file the lawsuit in the appropriate courthouse, ensuring all state deadlines are met.
- Negotiation and court proceedings: After the case is filed, your claim will move through the legal process, including the discovery phase. During discovery, both sides will exchange information and evidence related to your case. Your attorney will also enter into negotiations to determine if a fair and full settlement can be reached prior to trial. If a settlement cannot be reached, the case will be prepared for trial and presented before a judge and jury.
Saxenda Settlement and Payout Amounts
Saxenda settlement and payout amounts are expected to vary substantially depending on the individual circumstances of the case. There is no standardized settlement amount, however, ranges for serious injuries may be between $400,000 to $700,000. Factors that may influence the value of your case include the extent of your losses and the severity of your injuries.
An experienced attorney can help maximize compensation and negotiate for a fair settlement. Awareness of the full range of damages and required documentation is crucial. Without the assistance of legal counsel, you may end up settling for less than your case is worth.
Contact a Saxenda Lawyer to File a Claim
At King Law, our attorneys have decades of combined experience handling prescription drug-related claims. We are well-versed in navigating these complex lawsuits and will work to ensure you receive the compensation you deserve. If you took Saxenda and suffered gastroparesis or another serious gastrointestinal issue, contact our office to schedule a free consultation. Let us help determine your eligibility and take legal action.

