
People are filing Bard PowerPort lawsuits alleging the catheter device causes serious complications like fractures, infections, blood clots, hemorrhage, and heart punctures. The lawsuits claim Bard Access Systems, Inc., a subsidiary of Becton, Dickinson and Company, failed to warn patients of these risks. On May 24, 2023, MDL No. 3081 was filed to transfer cases to multi-district litigation. These lawsuits continue to be filed today.
Bard PowerPort Lawsuit Overview
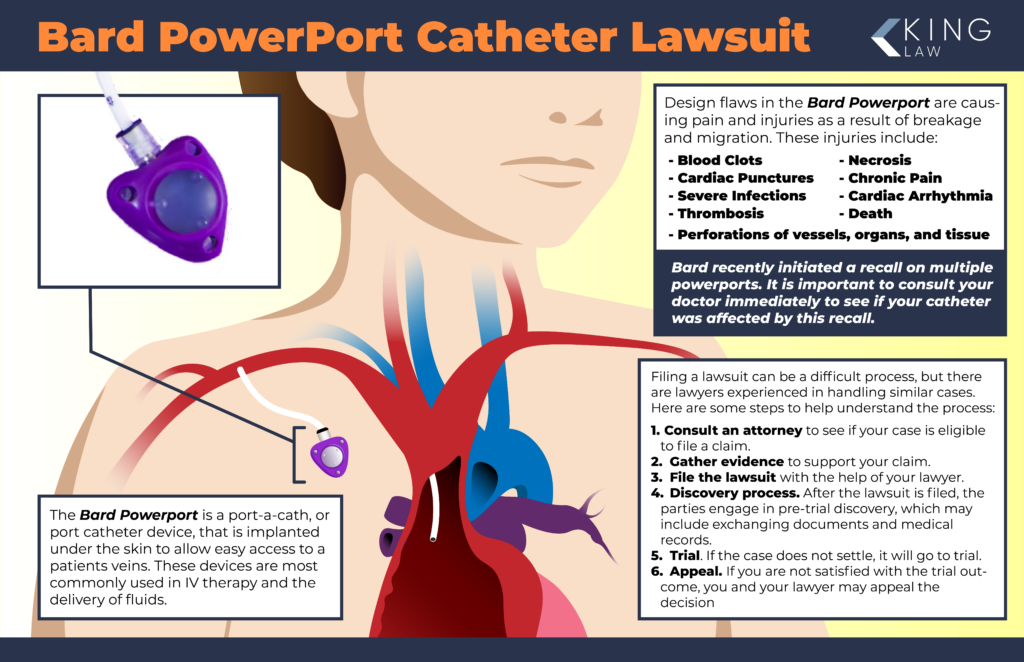
The Bard PowerPort is a port-a-cath, which is a port catheter device that is implanted under the patient’s skin to allow easy access to a vein. Port catheters are commonly used for medical IV therapy or delivery of fluids.
The Bard PowerPort was approved by the FDA in 2000 as an implanted port catheter device. However, design and manufacturing flaws make the device prone to breakage (fracturing) and migration, causing blood clots, serious injuries, such as cardiac punctures, and severe infections.
Due to injuries caused by the port-a-cath, Bard is facing product liability lawsuits, known in the legal community as the Bard PowerPort lawsuits. These lawsuits claim that patients who had a Bard PowerPort implant may have a higher risk of complications or injuries due to catheter failure.
In August 2023, the Bard lawsuits were consolidated as a federal “multi-district litigation”, before Judge David G. Campbell. The case is MDL 3081 and officially named “IN RE: BARD IMPLANTED PORT CATHETER PRODUCTS LIABILITY LITIGATION.”
If you, your family member, or loved one had a Bard PowerPort catheter device and subsequently suffered injuries or negative health effects due to fracture, migration, or other failures of the implant, you may be eligible for compensation. Contact an experienced product liability lawyer to get your case evaluated and prepared for a port catheter lawsuit.
Bard PowerPort and Port Catheter Lawsuit – 2025 Update
August 19, 2024: Louisiana Woman Files Bard PowerPort Lawsuit Alleging Serious Complications
On August 15, 2024, a Louisiana woman filed a Bard PowerPort lawsuit in the Multi-District Litigation in Arizona, alleging design defect, failure to warn, manufacturing defect, among other causes of action. In the case Davidson vs. Becton, Dickinson and Company, et al., the plaintiff was implanted with a PowerPort ClearVUE ISP Implantable Port on January 8, 2018, and subsequently experienced fracturing of the catheter device. A fracturing injury can be particularly dangerous, as the plastic components of the device can break free, migrate through the vein, and potentially reach the heart, leading to serious complications, including death.
August 1, 2024: Plaintiff Alleges Device Failure in Bard PowerPort Lawsuit
The complaint filed in Prentice v. Becton, Dickinson and Company; CR Bard Inc., Bard Access Systems provides a detailed overview of the Bard PowerPort port-catheter lawsuit. The twenty-two-page complaint was filed on April 14, 2023. The plaintiff alleges that the vascular access device was implanted on January 10, 2022, with the BardPort for chemotherapy in the treatment of breast cancer at a hospital in Phoenix, Arizona. A CT scan showed the port had fractured and moved. Emergency surgery was performed to retrieve the broken port and implant another one. The complaint alleges that the defendants knew the port might fracture from prior reports of failure. It also claims defective design, manufacture, and negligence. Additionally, the complaint alleges that ports have caused other injuries in other plaintiffs, like hemorrhage, perforation of tissue, cardiac arrhythmia, and pericardial tamponade.
July 24, 2024: PowerPort Case Numbers Continue to Climb
As of July 1, 2024, there are 299 pending actions in the PowerPort multidistrict litigation lawsuit. This is a large increase from the 69 pending actions in January of 2024. This means more people are taking action after being injured by faulty PowerPort devices.
July 14, 2024: Upcoming Case Management Conference Scheduled in Bard PowerPort Lawsuit
July 7, 2024: Upcoming Status Conference for Bard Implanted Port Catheter MDL
March 27, 2024: Key Study Highlights Risks in Bard PowerPort Lawsuit
One of the central issues in the Bard PowerPort lawsuit will be the types of injuries that the manufacturer (Bard) knew the PowerPort might cause. One important study the lawyers will likely argue about is titled “Complications after implantation of subcutaneous central venous ports (PowerPort).” The study, by Takatoshi Nakamura, published in the Annals of Medicine & Surgery in May 2017, indicated that infection was a significant risk of PowerPort use. The study also noted the risk of significant injury associated with rupture or fragment release from the catheter, the risk of thrombus, the risk of sepsis, and “catheter pinch-off syndrome.” Studies like this would appear to place the manufacturer on notice of specific risks associated with the use of the Bard PowerPort.
March 21, 2024: Court Approves PowerPort Lawsuit Plaintiff Profile Forms
This week Hon David G. Campbell, the judge overseeing the Bard PowerPort lawsuit, approved the Plaintiff Profile Form (PPF). This 30-page document must be completed by the attorney filing a lawsuit on behalf of their client. The information provided is used as reference by both the defendant and the court. The PPF contains 8 sections, including Background Information; Claim Information; Medical Background; Health Insurance Information; Prior Claim Information; Fact Witnesses; Identification of Documents; and Document Requests. The approval of the PPF by the Court is an important step in the litigation. One of the next major milestones will be the approval of a Master Complaint and a Short Form Complaint.
March 9, 2024: Slow growth of PowerPort Lawsuit a topic of discussion
In an updated Case Management Order by Judge Campbell, the Court addressed concerns about the slow pace of cases filed and the overall size of the MDL. To date there are approximately 115 lawsuits filed in this case, which is organized in the District of Arizona federal court. With an upcoming date to begin choosing candidates for trials, the defendants expressed concern that the case has not grown to the numbers predicted by the plaintiffs, and with such a small sample to choose from, any cases chosen for trial may not be fully representative of all of the potential plaintiffs. In a previous case involving inferior vena cava (IVC filters), which was also handled by Judge Campbell, the Court noted that litigation also started very slowly but began to pick up significantly, with more than 80% of the cases being filed after 18 months. In light of this, Judge Campbell has decided that the timeline for selecting trial candidates and discovery deadlines will remain as scheduled. The bellwether trial selection process will begin on April 1, 2024.
March 1, 2024: Bard PowerPort Lawsuit surpasses 100 cases
The Bard PowerPort lawsuit has been slow moving. Just over a half-dozen cases were added in February, with just 107 total cases filed in the multi-district litigation case. The PowerPort catheter lawsuit just became official in August of 2023, when all outstanding cases were transferred to federal court in the district of Arizona under Hon. David G. Campbell. With this case being brand new, expect several years of litigation before a full resolution.
February 26, 2024: Common Benefit Expense Fund Accounting Structure
Consistent with the Court’s case management order, the Court has appointed a Certified Public Accountant to establish an account and record-keeping practices for the Common Benefit Fund and Expenses. In a case such as the Bard PowerPort lawsuit, the attorneys selected to represent the interests of all plaintiffs, are entitled to a portion of an award or settlement for their years of work. Importantly, this percentage comes out of the fee of attorneys representing plaintiffs, it is not paid by the client. This account, managed by an independent third party, will ensure transparency for all expenses incurred by the plaintiffs’ leadership and eliminate commingling of funds with attorney’s accounts.
February 11, 2024: Litigation Continues for Bard PowerPort Lawsuit
Litigation continues for the Bard PowerPort lawsuit. According to the most recent filing from the Judicial Panel on Multidistrict Litigation (JPML), 99 lawsuits are pending consolidation.
January 20, 2024: Judges to rule on Bard PowerPort Reservoir Defects
The Judicial Panel on Multi-District Litigation (JPML) will soon rule as to whether post-reservoir defects will be added to the Bard PowerPort lawsuit. Their allegations deal with the use of polyoxymethylene, a type of plastic polymer, in the manufacturing of the port reservoir. That material can break down over time and can cause splintering or fracturing of the device. This PowerPort defect may cause infections and perforation of tissue and organs. The JPML will rule on this issue on January 25, 2024. If the panel decides to include the Bard PowerPort reservoir defects in the lawsuit, the master complaints will be amended to include it the addition.
January 1, 2024: Status Conference Set for Bard Powerport Port catheter lawsuit
After a very slow month of December where only a half dozen cases were filed in the lawsuit, we’ll get a progress update from the plaintiffs’ and defendant’s attorneys in a status conference to be held by Judge Campbell on January 8, 2023. With the Bard PowerPort litigation still in the early stages, fewer than 100 cases have been filed in this class action-style port catheter lawsuit.
>>> Read Past Bard PowerPort Lawsuit Updates Here
About the Bard PowerPort Lawsuit:
What is Wrong with the Bard PowerPort Device?
Common Bard PowerPort Complications and Injuries
Bard PowerPort Lawsuit Allegations
Manufacturer Recalls of Bard PowerPort Devices
How to File a Bard PowerPort Lawsuit
Eligibility Criteria for Filing a Bard PowerPort Lawsuit
PowerPort Settlement and Payout Amounts
What is Wrong with the Bard PowerPort Device?
Patients have experienced various degrees of PowerPort complications from Bard PowerPort usage. Lawsuits claim that the Bard PowerPort has a faulty design sometimes causing the catheter tube to crack, migrate and/or leak. These defects significantly increase the risk of infections, injuries, or incorrect/insufficient delivery of essential fluids or medication.
PowerPort catheter tubes are made of ChronoFlex, which is made from a mixture of silicone or polyurethane and barium sulfate. Barium sulfate is used to make the device visible on X-rays.
A PowerPort’s materials can degrade after they come in contact with the bloodstream. The barium sulfate used in the ports has been shown to degrade polyurethane and silicone after the port is inside the body. The barium sulfate particles don’t seem to fully integrate into the catheter polymer and air pockets tend to form, making the material fragile. Additionally, due to high concentrations of barium sulfate the material becomes less durable. Then, as the patient moves, the catheter bends and can fracture, subjecting the patient to the risks of thromboembolism and blood clots.
Catheter pieces can cause significant internal organ damage if they get into the bloodstream. Emergency surgery will then be required to remove fractured pieces and treat affected organs. The cost of removing a PowerPort is comparable to the cost of implanting it. The cost is believed to be around $10,000 without insurance.
Bard allegedly knew about PowerPort catheter fractures, migrations, and infections since its introduction in 2000. Bard’s failure to inform patients and medical providers and to recall the product in a timely fashion resulted in serious injuries and deaths and caused patients and family members to file lawsuits hoping for justice.
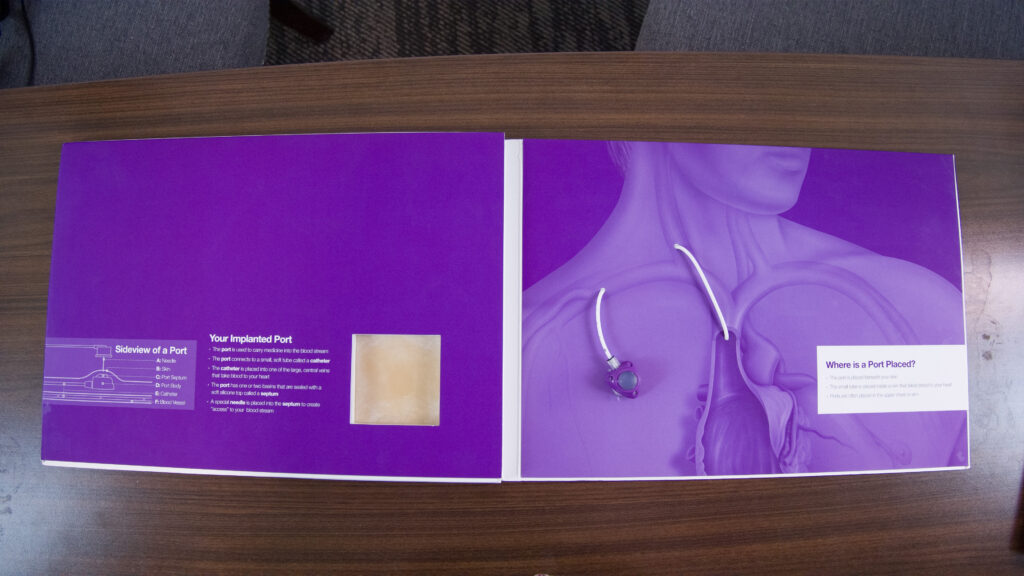
Bard PowerPort Design
Before we discuss injuries associated with Bard PowerPort catheters, it is important to understand their design and the materials used to make them.
The Bard PowerPort is an implantable catheter that is made up of two main parts:
- The injection port: a small, self-sealing port that is implanted under the skin close to a vein. It has a silicone septum so a needle may pass through the skin and then through the septum to inject medication directly into the reservoir for an IV treatment or blood transfusion.
- The catheter: a flexible tube that connects the injection port to a vein. The body of the catheter is made of polyurethane material and is radiopaque (it is visible on X-rays/MRIs). Some Bard PowerPort catheters are made using ChronoFlex (a family of biocompatible aromatic polycarbonate-based thermoplastic elastomers). ChronoFlex is supposed to be more durable than polyurethane.
| Component | Material |
|---|---|
| Injection port | Silicone |
| Catheter | Polyurethane or ChronoFlex |
Who Uses Bard PowerPort Catheters?
The Bard PowerPort is used to deliver medications, fluids, and blood transfusions. It is often used in cancer patients who are receiving chemotherapy or other cancer therapies. PowerPort is also used in patients with chronic kidney disease, people who suffer from irritable bowel disease (IBD), or patients who need regular blood transfusions.
These catheters allow medical professionals to have direct and repeated access to a vein in the body’s vascular system. People suffering from other medical conditions may benefit from these devices.
These ports can increase a patient’s comfort during medical treatments. The ports also reduce the risk of tissue damage. Once the patient heals from device insertion, they can then swim and bathe normally, without an increased risk of infection.
Although these ports are helpful for many people, port insertion and use can have serious side effects. One study found that 6% of patients who had implanted PowerPorts experienced a post-surgical complication, including infection, catheter pinching, or port extravasation (extra-vascular leaking).
PowerPorts for Chemotherapy
Port catheters are a common way to deliver chemotherapy drugs to cancer patients. Cancer patients often endure multiple blood draws, infusions, and injections. A port catheter allows doctors to administer these treatments and tests. Doctors can also use the ports to provide IV fluids and administer blood products such as platelets and plasma. The ports make administering these treatments easier and provide greater comfort to patients during such treatments. The catheters are supposed to protect a patient’s veins from injuries caused by repeated access.
PowerPorts can also be used to give patients IV contrast for imaging scans. Cancer patients often have MRIs or CT scans, so the ports give medical professionals another way to inject contrast for these tests.
PowerPorts for IBD and Other Conditions
Because PowerPorts access the body’s vascular system, they are used for a variety of reasons. People with bowel disease or severe infections may also be good candidates for port catheters. Because medical professionals can use the ports to administer IV fluids, blood products, and parenteral nutrition (feeding someone through a vein), they are used for a variety of conditions, including IBD.
Common Bard PowerPort Complications and Injuries
Patients have suggested that the PowerPort device is less durable than it should be. A growing number of lawsuits point out a flaw in the design of the ChronoFlex catheters that results in the following Bard PowerPort problems:
- Catheter fracture injuries
- Catheter-related infections
- Catheter migration
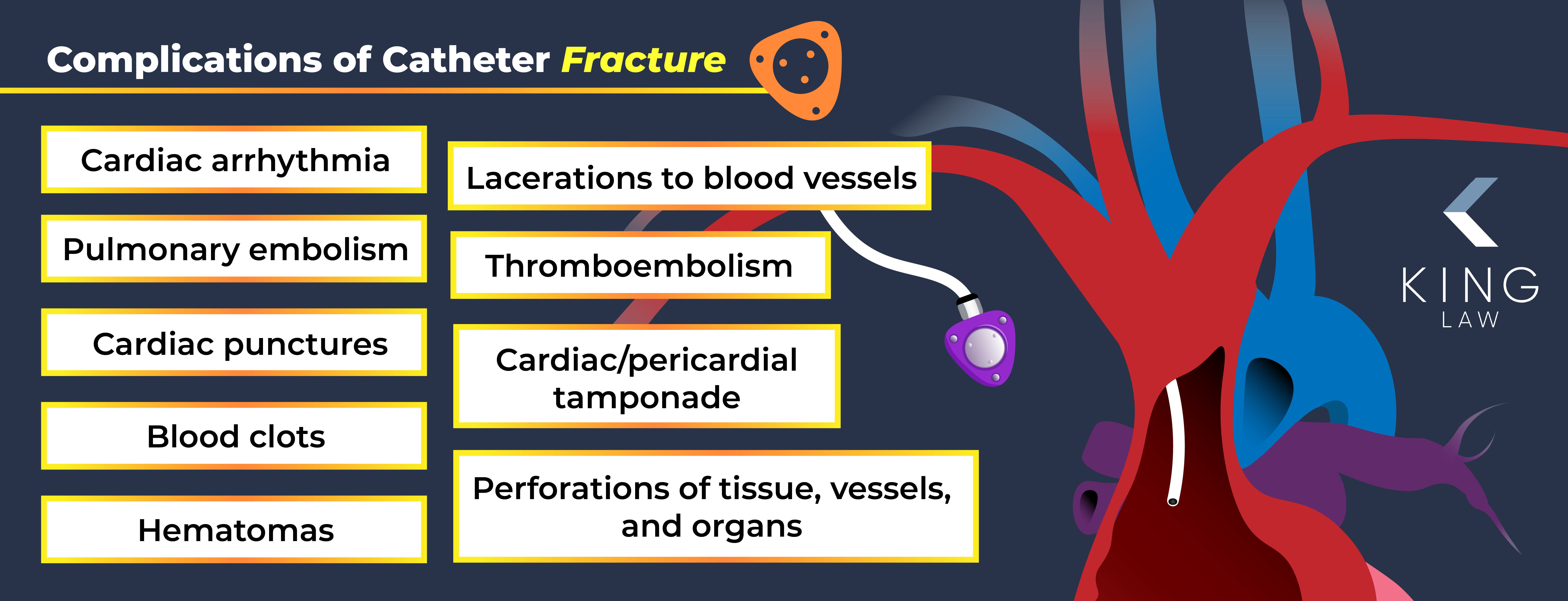
Bard Catheter Fracture Injuries
The PowerPort catheter tube is prone to fracturing, which can lead to small pieces of plastic breaking away and getting into the vascular system, potentially leading to life-threatening complications. This means some PowerPort patients could experience the following injuries:
- Cardiac arrhythmia
- Pulmonary embolism
- Cardiac punctures
- Blood clots
- Hematomas
- Lacerations to blood vessels
- Cardiac/pericardial tamponade (pressure caused by an accumulation of fluid in the area around the heart)
- Thromboembolism
- Perforations of tissue, vessels, and organs (e.g., heart or lungs)
Bard catheter failure can also result in severe and persistent pain. Emergency surgery is often required to remove fractured pieces and treat affected organ systems.
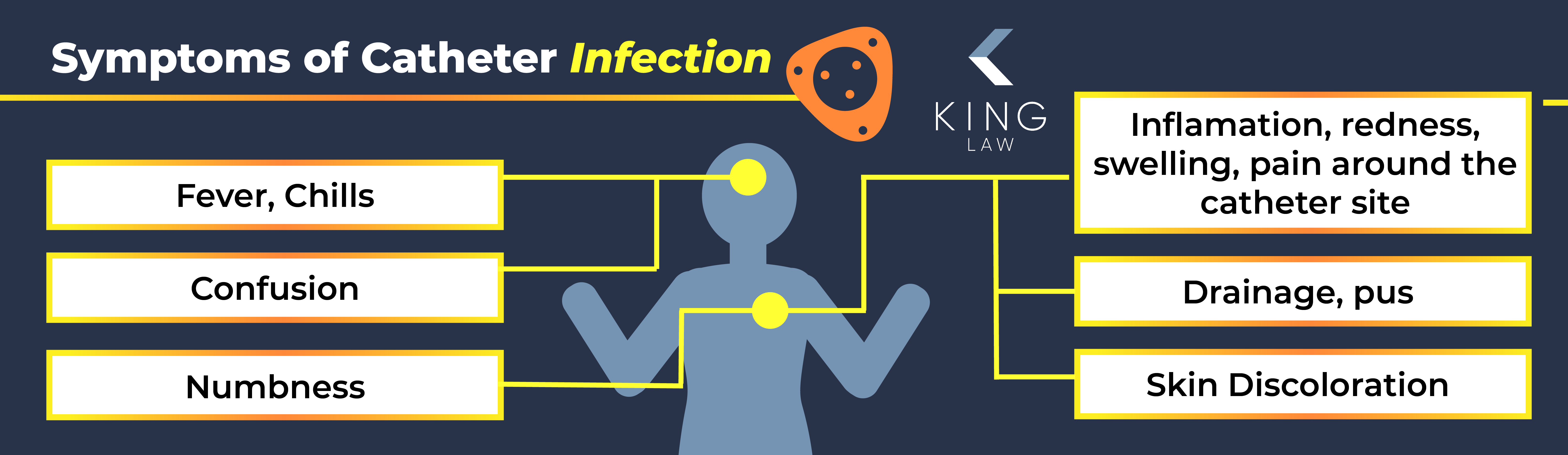
Bard Catheter Infection Injuries
Patients with a Bard catheter may develop infections due to bacteria entering broken areas of the PowerPort. Common symptoms of an infection include:
- Fever
- Chills
- Inflammation, redness, swelling or pain around the catheter site
- Drainage or pus
- Skin discoloration
- Numbness
- Confusion
Complications from catheter infection can be serious and even life-threatening.
Infection around the catheter site can lead to bloodstream infections, severe swelling, and even necrosis of tissues around the PowerPort.
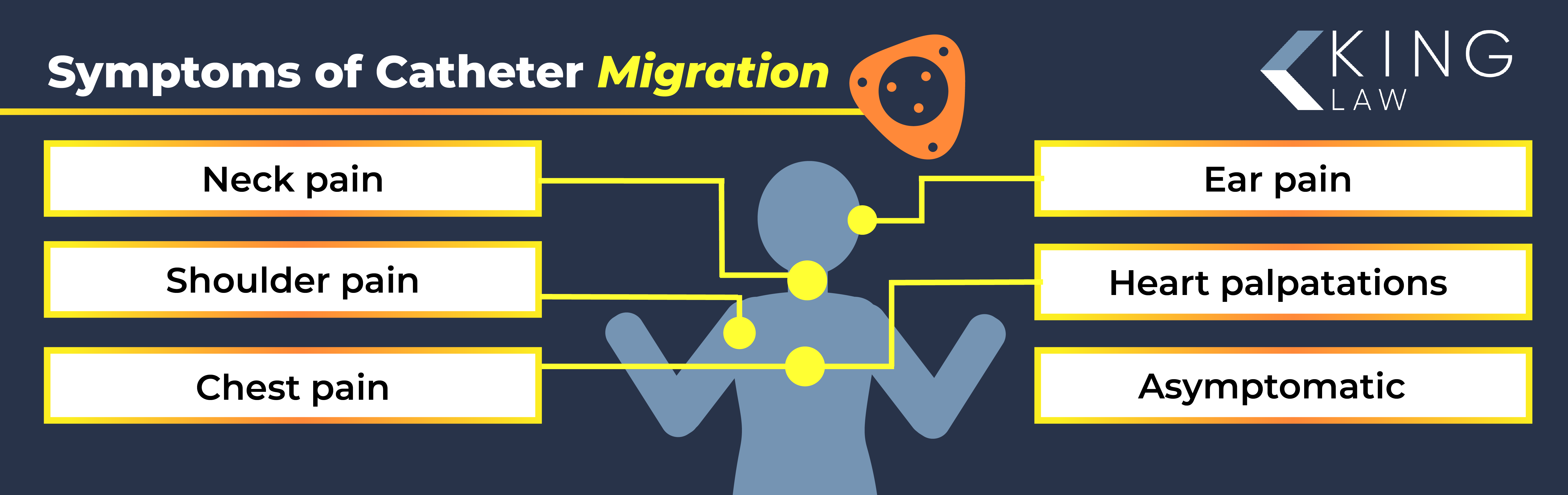
Bard Catheter Migration
Catheter disconnection and migration are rare but serious complications associated with Bard PowerPort. It can lead to side effects such as venous thrombosis and neurologic problems.
Patients experiencing catheter migration can be asymptomatic, which makes diagnosis and treatment more difficult. The most common port-a-cath migration symptoms include:
- Neck pain
- Shoulder pain
- Chest pain
- Ear pain
- Heart palpitations
Entire catheter dislodgement is even more rare, but unfortunately possible and requires emergency surgery.
Catheter migration can lead to obstruction of blood flow, infection and organ damage.
Chemo Port Injuries
PowerPorts have been shown to rupture when patients are receiving IV contrast for CT scans or MRIs. Shortly after the PowerPort came to market, the FDA received more than 250 adverse event reports about power port catheters. These reports documented that some power injector ports ruptured when contrast media was given as part of CT or magnetic imaging.
This puts cancer patients and other people who need frequent imaging tests at risk for port rupture and the associated side effects.
Bard PowerPort Lawsuit Allegations
The Bard PowerPort lawsuits claim that the devices are defective, creating possible infections and catheter failures that can lead to thrombosis, organ damage, and even death. The lawsuits allege that Bard Access Systems, Inc., and its parent company, Becton Dickinson and Company, the manufacturers of the PowerPort, knew about the risks associated with the device, but failed to inform patients and medical professionals.
The lawsuits specifically note that the PowerPort is susceptible to fractures and infections. Some of the specific injuries that have been reported in connection with the PowerPort include:
- Thrombosis
- Damage to veins and blood vessels
- Necrosis
- Blood clots
- Cardiac/pericardial tamponade
- Cardiac arrhythmia
- Perforations of vessels, organs, and tissue
- Chronic pain
Manufacturer Recalls of Bard PowerPort Devices
Bard recently initiated a recall for multiple lots of PowerPort. The following products were affected:
- PowerPort Implantable Port With Attachable 9.6 F Open-Ended Single-Lumen Venous Catheter REF: 1709600, Air Guard, With suture Plugs, (01)00801741026720;
- PowerPort Implantable Port With Attachable 9.6 F Open-Ended Single- Lumen Venous Catheter, REF: 1709601, Air Guard, Without Suture Plugs, (01)00801741026737;
- PowerPort Implantable Port with Pre-Attached 9.6F Open-Ended Single-Lumen Venous Catheter, REF: 1759600 Air Guard, With Suture Plugs, (01)00801741026850
If you have a Bard PowerPort implant, it is important to consult your doctor immediately to see if your catheter is affected by the recall. You can also check the FDA’s website for a list of all recalls of Bard PowerPort devices.
The class-2 recall opened in March of 2020 and terminated in February of 2022. The FDA issued the recall because of an incorrect barb housed within the device. If you were affected by the recall, you should contact an attorney to discuss your options.
How to File a Bard PowerPort Lawsuit
If you or someone you know has been injured by a Powerport, please contact one of our experienced attorneys. We offer free case reviews. We will help you navigate tasks such as:
- Collecting evidence to support your claim, including medical records and witness testimonies
- Determining state-specific deadlines and statute of limitations
- Handling settlement negotiations
- Setting the case for trial if a favorable settlement cannot be reached
How Much Time Do You Have to File a Lawsuit?
Because of statutes of limitations, it’s important to consult with a lawyer promptly. If you have been harmed by a PowerPort catheter, you should contact an attorney as soon as possible after your injury. Each state has its own laws about how much time can pass between an injury and a lawsuit filing. Our attorneys can help you understand which state you can file your case in and states’ filing deadlines. They will help you achieve the best possible outcome.
What to Expect During the Legal Process
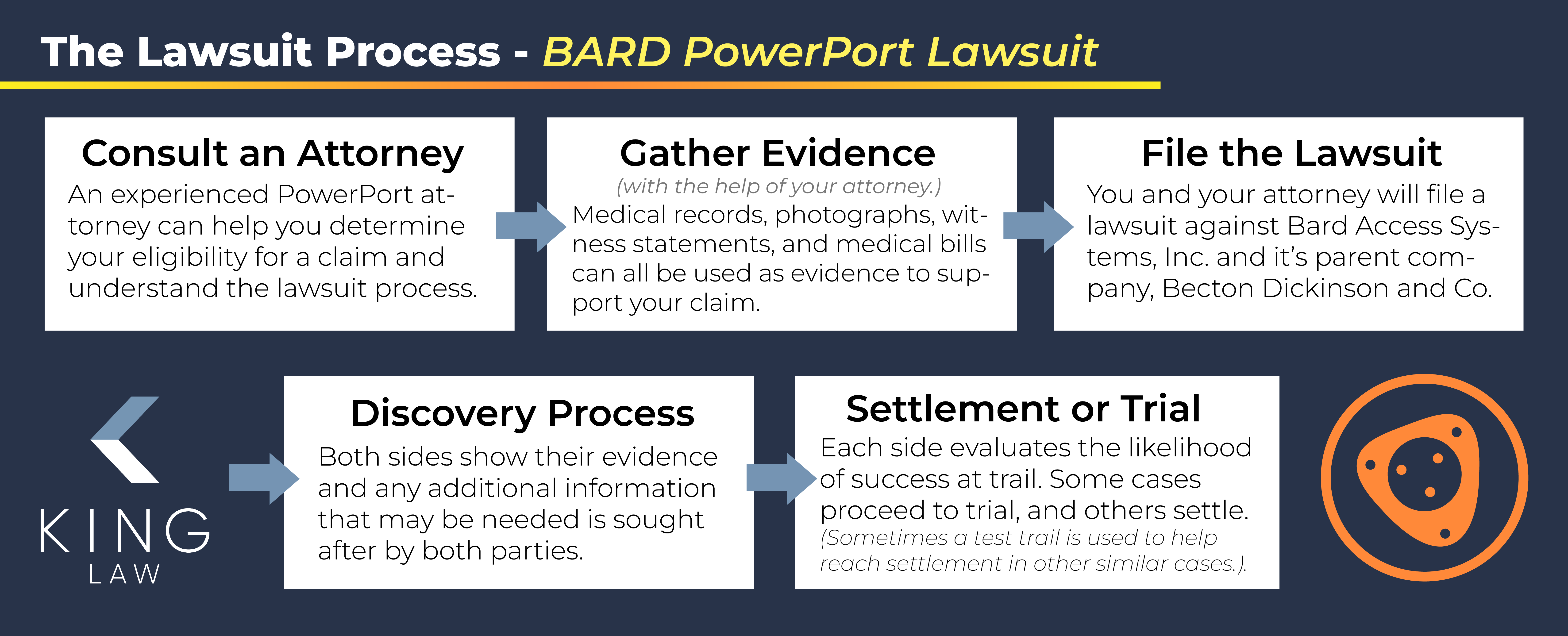
Our lawyers will work with you throughout the legal process for filing a Bard PowerPort lawsuit. We recommend working with a lawyer who has experience handling similar cases. Here are the steps involved:
- Consult an attorney. An experienced Bard PowerPort attorney can help you evaluate your case and determine if you are eligible to file a claim. They can also help you understand the legal process and timeline.
- Gather evidence. You will need to gather evidence to support your claim. This usually includes medical records, photographs, witness statements, and medical bills.
- With the help of your lawyer, file a lawsuit. Once you have gathered evidence, you will need to file a lawsuit against Bard Access Systems, Inc., and its parent company, Becton Dickinson and Company.
- Discovery process. Both sides gather evidence and documentation. Your attorney will support you throughout this process and advise what documentation will best support your case, such as medical records, bills, and proof of payment.
- Settlement or trial. You and your attorney may choose to pursue a settlement. If the case doesn’t get settled, it could go to trial. At trial, a jury will decide whether Bard is responsible for your injuries and subsequent suffering/loss of income/loss of consortium/etc. If the jury finds Bard responsible then they will determine the payout amount you are entitled to.
- Appeal. If you are not satisfied with the outcome of the trial, you and your lawyer may appeal the decision.
Eligibility Criteria for Filing a Bard PowerPort Lawsuit
Individuals who have received a diagnosis for an adverse health condition after receiving a PowerPort catheter may be entitled to take legal action.
Eligibility criteria for filing an PowerPort lawsuit include:
- Proof that you had a Bard PowerPort implantable catheter inserted
- A diagnosis of an adverse health condition, such as cardiac arrhythmia; pulmonary embolism; cardiac punctures; blood clots; hematomas; lacerations to blood vessels, cardiac/pericardial tamponade; thromboembolism; or perforations of tissue, vessels, and organs (e.g., heart or lungs)
- An emergency room visit, hospitalization, or similar medical visit related to the condition caused by the faulty PowerPort device
It is critical to consult with a PowerPort lawyer to determine if you meet the criteria requirements and for state-specific statutes of limitations that may affect your case.
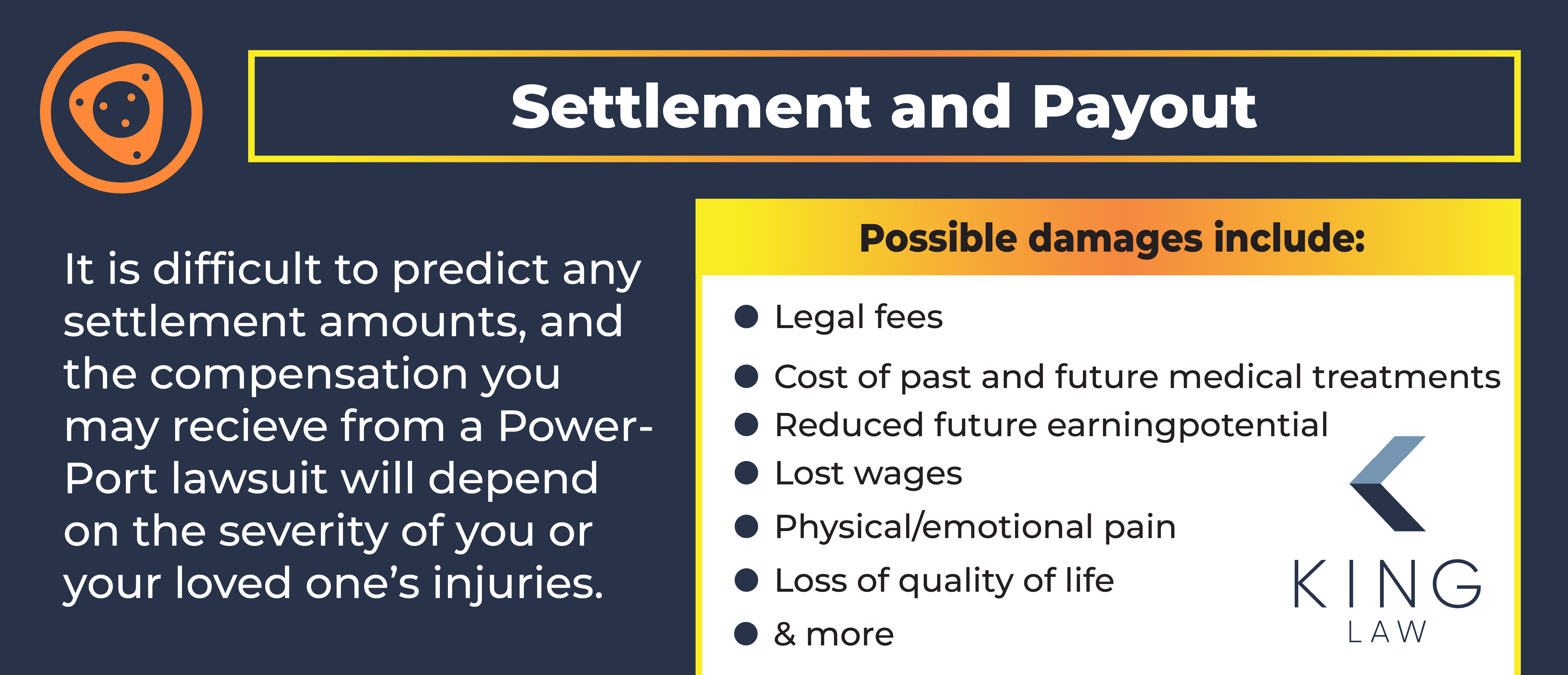
PowerPort Settlement and Payout Amounts
It is difficult to predict the Bard settlement amounts. Further, the amount of compensation you could receive from a PowerPort lawsuit settlement will depend on the severity of your/your loved one’s injuries. It is also important to note that compensation or even favorable outcomes of the trial are not guaranteed.
Possible damages may include:
- The cost of past and future medical treatments
- Legal fees
- Lost wages
- Reduced future earning potential
- Decreased quality of life
- Physical and emotional pain and suffering
- The loss of a loved one
PowerPort Lawsuit Payout Amounts
If your case goes to trial, the specific amount of compensation you receive will depend on the individual circumstances of your case. An attorney will work with you to understand and document the losses you have experienced. For a free case evaluation, consult our team of experienced product liability and personal injury lawyers.
Factors That Determine Settlement Amounts
There are multiple factors that would affect the amount a patient receives from a successful settlement. If you were harmed by a PowerPort device, your individual factors and hardships would be considered. It’s important to consult an experienced attorney who can document the losses you experienced and fight for a fair and full settlement.
Contact a Port Catheter Injury Lawyer
At King Law, our PowerPort lawyers provide dedicated representation for individuals who have suffered adverse health conditions as a result of having a port-a-cath implanted to receive medical treatment. We have years of experience advocating on behalf of those injured by defective medical products.
We are a team of experienced personal injury lawyers with a strong commitment to our clients. Call us today for a free case evaluation to see if you qualify for the Bard PowerPort lawsuit.

Latest News on Bard PowerPort
December 3, 2023: Court Approves Short Form Complaint in Powerport Port Catheter “Class Action” Lawsuit
On November 30, 2023, Judge Campbell, who is overseeing the Bard PowerPort Port Catheter “class-action” style lawsuit in Arizona Federal District Court, issued an order approving the short form complaint. This document, which is a condensed version of the long-form complaint, details the allegations made by the plaintiffs that the devices caused serious injuries such as splintering, blood clots, organ perforation, infections and others. The complaint also outlines the specific products that are the basis of the lawsuit. After a plaintiff’s attorney builds the case for their client, they will file the short-form complaint with the court, initiating the lawsuit.
November 21, 2023: Bard Files Motion to Strike Allegations from PowerPort catheter Master Complaint
The defendants (C.R. Bard) in the PowerPort Catheter class action lawsuit are asking the judge overseeing the case to strike certain allegations in the proposed master complaint. A master complaint is a document which outlines in great detail the allegations and injuries which are the basis of the lawsuit. Bard claims they improperly expand the scope of the multi-district litigation (MDL) lawsuit. The defendant contends that the plaintiffs are attempting to add new allegations that are not relevant to the focus of the lawsuit. The argument pertains to whether certain components of the product are defective. The defendants are asking a judge to rule on the issue prior to responding to the proposed master complaint. The judge responded and noted that he will take the request under consideration.
October 1, 2023: Court holds initial conference in Bard PowerPort Lawsuit
Recently, Hon. David G. Campbell, the judge in charge of the Bard PowerPort Lawsuit held a conference to discuss motions and responses filed by both parties. Additionally, Court announced it will hold the first Case Management Conference on November 16, 2023, in federal court for the District of Arizona, where the case has been consolidated. The Court is also expected to make a decision on the selection of a Plaintiff’s leadership committee in the coming weeks. Lawyers selected for the leadership positions will be responsible for representing the best interests for all plaintiffs.
September 14, 2023: Leadership Structure Proposed in Bard PowerPort Lawsuit
Nearly 40 plaintiffs’ attorneys have just been proposed to hold leadership positions in the ongoing Bard PowerPort lawsuit. This proposal nominates the following professionals: 3 attorneys to serve as Co-Lead Counsel; 11 attorneys to serve on the Executive Counsel or Liaison Counsel; 13 attorneys to serve on the Steering Committee; and, 12 attorneys to serve as Sub-Committee Members. It will be the responsibility of leadership committee members to carry out specific functions throughout the litigation process. Some of these functions may include, but are not limited to, conducting investigations and interviews that have a shared interest in all claims, preparing and presenting arguments before the Court, and engaging in settlement discussions that lay the groundwork for resolving all claims related to Bard PowerPort lawsuit injuries. These attorneys are obligated to act in the best interest of every individual that has brought a claim for injury against Bard PowerPort, and that each individual claim will remain separate and distinct from one another. As a component of organizing these lawsuits, it is anticipated that the Judge will order the plaintiffs and the defendant to select several “test” cases for trial. These so-called “bellwether” trials will serve as a means of assessing how juries might react to specific injuries, evidence, and testimony. The results of these test trials will form the groundwork for later settlement negotiations. This litigation is still in the very beginning stages of a process that is expected to take several years.

