We are currently not accepting new Gardasil cases at this time.
In 2006, the first generation of Gardasil was approved to prevent infection of the Human Papillomavirus (HPV). The manufacturer of Gardasil, Merck & Co., has recently come under scrutiny for allegedly fast-tracking the approval process and overlooking dangerous side effects related to the use of the drug. Gardasil-9 (the current generation) is approved for use in children as young as nine.
Merck & Co is now facing several lawsuits related to the use of the HPV vaccine. Lawsuits allege that the company concealed the known health risks associated with the use of the drug and misrepresented its efficacy against cervical cancer. Reported complications of the drug include postural orthostatic tachycardia syndrome (POTS), autoimmune disorders, premature ovarian failure, and in some instances, death.
Victims who suffered debilitating health complications may be eligible for compensation through a Gardasil vaccine lawsuit and are strongly encouraged to contact an attorney. King Law provides dedicated representation for Gardasil victims and their families. We are currently investigating cases for individuals that received the vaccine in 2019 or later. Contact our office today to schedule a free, no-obligation consultation.
On this page:
Gardasil Vaccine Lawsuit Update
Gardasil Issues and Controversies
Gardasil Manufacturer: Merck & Company
Health Risks Linked to Gardasil Vaccine
Manufacturer Recalls and Discontinuation of Gardasil Vaccine
Gardasil Payout and Settlement Amounts

Gardasil Vaccine Lawsuit Update
March 12, 2025: RFK’s Confirmation as Secretary of Health Could Impact Active Gardasil Lawsuits
Robert F. Kennedy Jr. has been confirmed by the U.S. Senate as the U.S. Secretary of Health and Human Services. Interestingly, Mr. Kennedy worked as a lawyer and helped bring early Gardasil cases against the manufacturer. Many have speculated about whether Kennedy’s role in government might have bearing on the outcome of the Gardasil lawsuits.
December 10, 2023: Gardasil Lawsuit Trial Update
The court overseeing the Gardasil lawsuits recently published an update regarding bellwether trial status. Bellwether trials are a type of test trial held to gage how juries will respond to evidence and ultimately rule in certain types of cases. The court noted that many plaintiffs and their parents have been interviewed, or deposed, and more are yet to come. There was also some question from some parents about being required to reveal their own family medical history in these interviews.
October 11, 2023: Status Conference held in Gardasil Vaccine Lawsuit
Recently, the judge overseeing the Gardasil Lawsuit in federal court, held a status conference with the parties. Three main topics were discussed including the status of a motion filed by the Plaintiff’s regarding documents and testimony, Discovery limits, and Bellwether case updates. Parties have agreed on 16 bellwether cases and discovery continues to be exchanged between parties. Many of the bellwether cases have been amended by the plaintiff’s and it is possible that some cases may be replaced if dismissed.
August 2023: The second FRCP 30(b)(6) deposition in the Gardasil Products Liability MDL was scheduled for August 2, 2023.
July 2023: In July 2023, a Joint Status Report for the July 25 pretrial conference in the Gardasil Products Liability MDL was filed. The Joint Status Report indicated that both parties proposed canceling the July 25 status conference. The Joint Status Report explained that the proposed cancellation was due to the scheduling of the FRCP 30(b)(6) depositions, as well as meetings to review Merck’s privilege logs and the production format of materials. The first of two depositions was scheduled for July 27, 2023.
>>> Read Past Gardasil Vaccine Lawsuit Updates Here
What Is the Gardasil Vaccine?
The Gardasil vaccine was developed to prevent the spread of the human papillomavirus (HPV). According to the U.S. Centers for Disease Control and Prevention (CDC), HPV is the leading sexually transmitted disease in the United States. While many of the over 150 strains of HPV are innocuous, there are some that can cause cancer.
The first-generation Gardasil protected against four strains of HPV. Gardasil-9 (the current generation) reportedly provides protection against nine different potentially cancer-causing strains of the virus.
Merck & Co.’s Gardasil-9 vaccine is approved for children as young as nine. It is generally administered to children and adults between the ages of 9 and 26 to protect against cancer-causing strains of the human papillomavirus (HPV).

Gardasil Issues and Controversies
To ensure the safety of consumers, clinical trials must be conducted under stringent regulatory practices, and there must be clear, unambiguous communication about the efficacy and safety of the drug.
The controversy surrounding the Gardasil vaccination began almost immediately. Prior to receiving FDA approval, it is alleged that Gardasil manufacturer Merck & Co. conducted clinical trials in a deceptive and unscientific manner. Litigation also asserts that the company fast-tracked approvals without adhering to standard testing methods placing into question the credibility of the results of the clinical trials.
In addition to questions regarding the testing procedures, such as whether any participants were given a true saline placebo, it is also believed that Merck made material misrepresentations as to the efficacy of the vaccine. Lawsuits filed against the manufacturer question whether the vaccine has proven effective at preventing cervical cancer and other forms of cancer.
Gardasil Manufacturer: Merck & Company
Merck & Co. (“Merck”) is one of the largest pharmaceutical companies. It produces a wide range of medicines and vaccines, including the Gardasil vaccine. As the developer of the first-generation Gardasil and the current-generation Gardasil-9, Merck is the primary defendant in all Gardasil-related lawsuits.
Gardasil was developed by Merck with the intent to protect against HPV-related cancers. However, it is believed that in the company’s rush to obtain FDA approval, they may have failed to ensure the safety and efficacy of the vaccine. Despite receiving reports of serious side effects and even deaths potentially related to the drug, Merck continued to promote its safety and continued to lobby for the vaccine to become mandatory.
FDA Approval of the Gardasil Vaccine
The U.S. Food and Drug Administration (FDA) is the federal regulatory agency responsible for ensuring the safety and efficacy of drugs and vaccines distributed in the United States. Consumers nationwide rely on the FDA to maintain a rigorous approval process.
When an unsafe product receives approval because the FDA failed to complete a comprehensive evaluation, it erodes public trust in other life-saving drugs and vaccines. Thorough and transparent procedures at all levels of the approval process are critical.
In 2006, the FDA approved the use of Gardasil as a vaccine in females between the ages of 9 and 26 years of age to prevent diseases related to the four strains of the human papillomavirus. Merck received expedited approval from the FDA amidst allegations of deceptive research and misleading clinical trials.
In 2014, the FDA approved Gardasil-9 (also manufactured by Merck). Gardasil-9 is marketed as preventing diseases related to nine of the most common types of HPV. Gardasil-9 is now the only HPV vaccine distributed in the country.
Misleading Trials and Marketing of the Gardasil Vaccine
Lawsuits filed against Gardasil manufacturer Merck allege that the pharmaceutical giant manipulated its clinical trials of the vaccine to ensure a fast-tracked FDA approval. It is believed that the company overinflated the benefits of the drug compared to the potential risk of serious side effects. Merck is further accused of failing to disclose key ingredients during its Gardasil clinical trials.
Shortly after Merck received initial FDA approval of the vaccine, it engaged in a multi-faceted lobbying campaign. Marketing related to Gardasil urged parents to vaccinate their young children against HPV, a sexually transmitted disease. Not only is Merck accused of misleading marketing strategies but also of mislabeling the drug as a “cervical cancer vaccine.” It is alleged that Gardasil clinical trial results did not support these findings.
Political Lobbying of Mandatory Gardasil Vaccine
In their efforts to increase sales of the vaccine, Merck went on an aggressive campaign to make the drug mandatory for young girls. The drug manufacturer lobbied state legislatures providing the majority of the scientific information about the vaccine to lawmakers.
Additionally, Merck employed the use of local political consultants, prominent physicians, and public relations firms to further their agenda and increase the drugmaker’s bottom lines. To leverage its position, Merck also allegedly provided financial incentives to legislatures and made contributions to political campaigns.
Merck’s lobbying led to the passage of laws in states like Texas, where Governor Rick Perry received significant criticism for requiring girls to receive the vaccine before entering the 6th grade. In some cases, preteens were allowed to consent to the vaccination without parental knowledge or approval. After considerable backlash, the company was forced to end those lobbying efforts and refocus its campaigns.
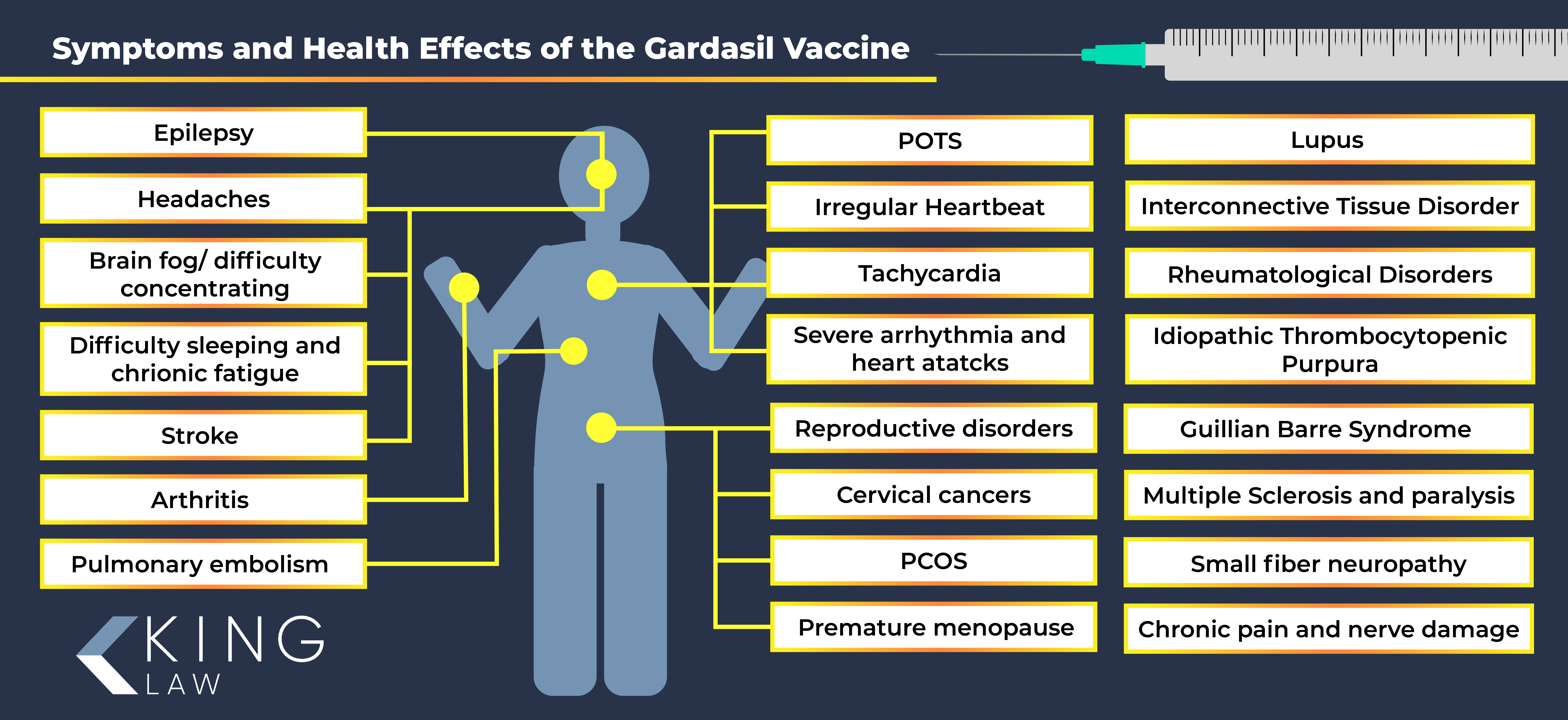
Side Effects of Gardasil
The Vaccine Adverse Event Reporting System (VAERS) is an early warning system that helps to monitor for adverse reactions to a vaccine that has been approved for use by the FDA. It is widely reported that thousands of people filed complaints of adverse reactions through VAERS related to Gardasil. Complications and side effects of Gardasil varied, but there are believed to be deaths linked to the use of the vaccine.
Gardasil vaccine side effects may include:
- Autoimmune disorders such as lupus and arthritis
- Interconnective Tissue Disorder and Rheumatological disorders
- Idiopathic Thrombocytopenic Purpura (ITP)
- Neurological disorders including epilepsy and Guillain Barre Syndrome
- Brain fog or difficulty concentrating
- Multiple sclerosis and paralysis
- Small fiber neuropathy
- Cardiovascular issues including POTS (Postural Orthostatic Tachycardia Syndrome)
- Irregular heartbeat or tachycardia
- Severe arrhythmia and heart attacks
- Pulmonary embolism and stroke
- Reproductive disorders such as premature ovarian failure (POF)
- Cervical cancer
- Polycystic Ovary Syndrome (PCOS) and premature menopause
- Chronic pain, severe headaches and nerve damage
- Difficulty sleeping and chronic fatigue
In some cases, it is alleged that the Gardasil side effects resulted in severe health complications including placing a person in a coma, causing acute disseminated encephalomyelitis or even proved deadly.
Lawsuits filed against Gardasil indicate that some of the adverse effects of the vaccine may be related to its ingredients. The drug contains controversial ingredients such as AAHS and Polysorbate 80, which have been linked to an increased risk of cognitive impairment and motor issues.
Gardasil Side Effects Long-Term (~10 years and later)
One of the biggest concerns related to the use of the vaccine is the long-term side effects. People indicate that they suffer from Gardasil side effects years later.
Long-term side effects of Gardasil include but are not limited to:
- Autoimmune disorders such as fibromyalgia, dysautonomia, and Guillain–Barré syndrome.
- Reproductive Disorders, including premature ovarian failure and primary ovarian insufficiency.
- Neurological disorders such as chronic fatigue syndrome, migraines, and brain fog.
In addition, Gardasil side effects 10 years later have been tied to persistent gastrointestinal discomfort, joint pain, and widespread neuropathic pain.
Health Risks Linked to Gardasil Vaccine
There are a number of health risks linked to the use of the Gardasil vaccine, based on reports through VAERS and pending litigation against the manufacturer. Contrary to Merck’s claims, evidence indicates that Gardasil may increase the risk of cervical cancer.
Gardasil health risks include:
- Increased risk of cancer
- Autoimmune disorders including postural orthostatic tachycardia syndrome and chronic inflammatory demyelinating polyneuropathy
- Premature ovarian failure
- Fibromyalgia
- Chronic regional pain syndrome
- Chronic fatigue syndrome
There is also a link between the vaccine’s controversial ingredients like Hydroxyphosphate Sulfate (AAHS) and autoimmune disorders, including Guillain–Barré syndrome and multiple sclerosis.
Manufacturer Recalls and Discontinuation of Gardasil Vaccine
On December 16, 2013, Merck issued a voluntary recall of the Gardasil vaccine over potential contamination. As reported by the CDC, the recall was initiated because vials of the vaccine potentially contained glass particles caused by breakage during the manufacturing process.
In October 2020, Merck issued a second recall due to prefilled syringes of the vaccine being stored at an inappropriate temperature. Since then, there have been no further Gardasil recalls despite demands.
It is important to note that the vast majority of lawsuits are based on the fact that the drugmaker failed to sufficiently inform patients, doctors, and parents about the potential harm from the use of the vaccine.
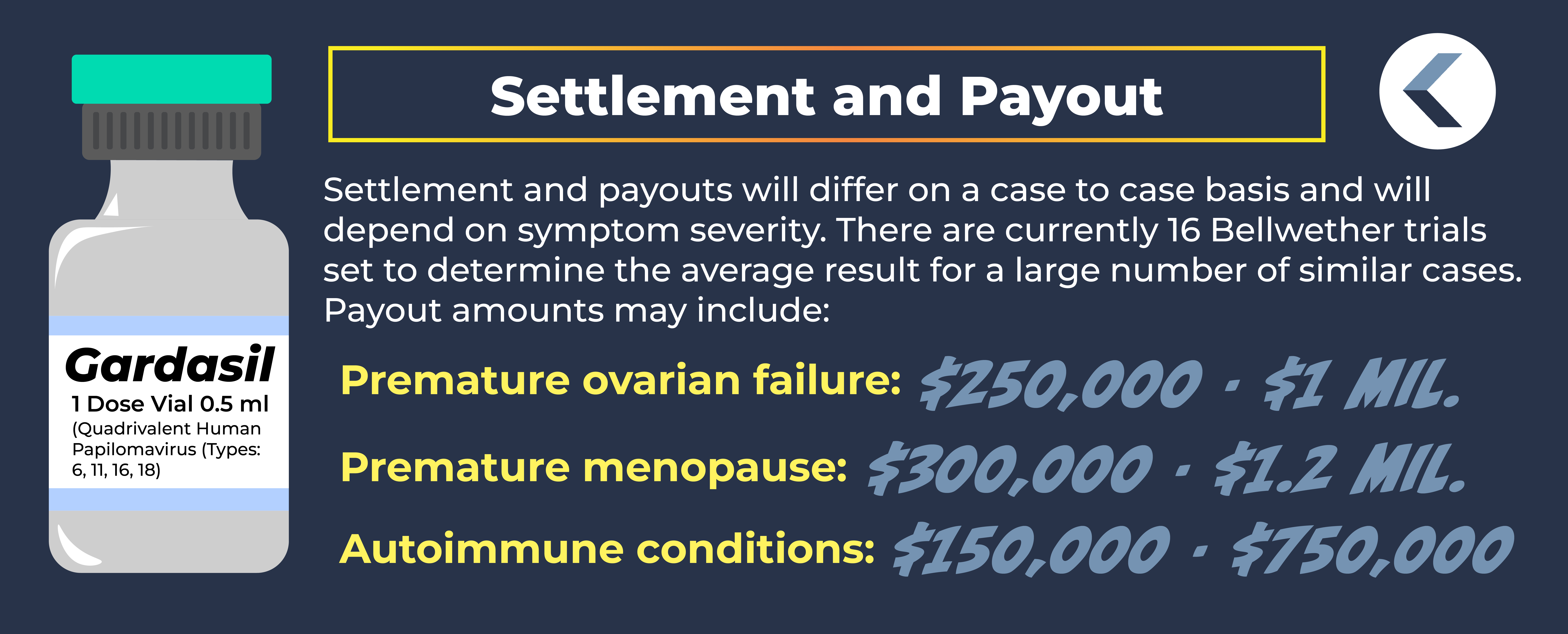
Gardasil Payout and Settlement Amounts
As more potential victims come forward, and the Multidistrict Litigation filed against Merck gets underway, there will be a clearer understanding of what the anticipated Gardasil payout and settlement amounts may be.
Gardasil lawsuit payout amounts may include:
- Premature ovarian failure: an estimated value range of $250,000 to $1,000,000.
- Premature menopause: an estimated value range of $300,000 to $1,200,000.
- Autoimmune conditions such as Guillain-Barre syndrome and POTS: an estimated value range of $150,000 to $750,000.
Currently, there are 16 Bellwether trials set in the Gardasil MDL. These trials, which will be narrowed down, are expected to start in late 2024 or early 2025. Bellwether trials refer to a small number of preliminary trials used to forecast the results of a large number of similar cases.
Gardasil lawsuit settlements may be limited by the Vaccine Act as it may have you file a claim through the National Vaccine Injury Compensation Program. Compensation through the program is usually less than filing a personal injury lawsuit.
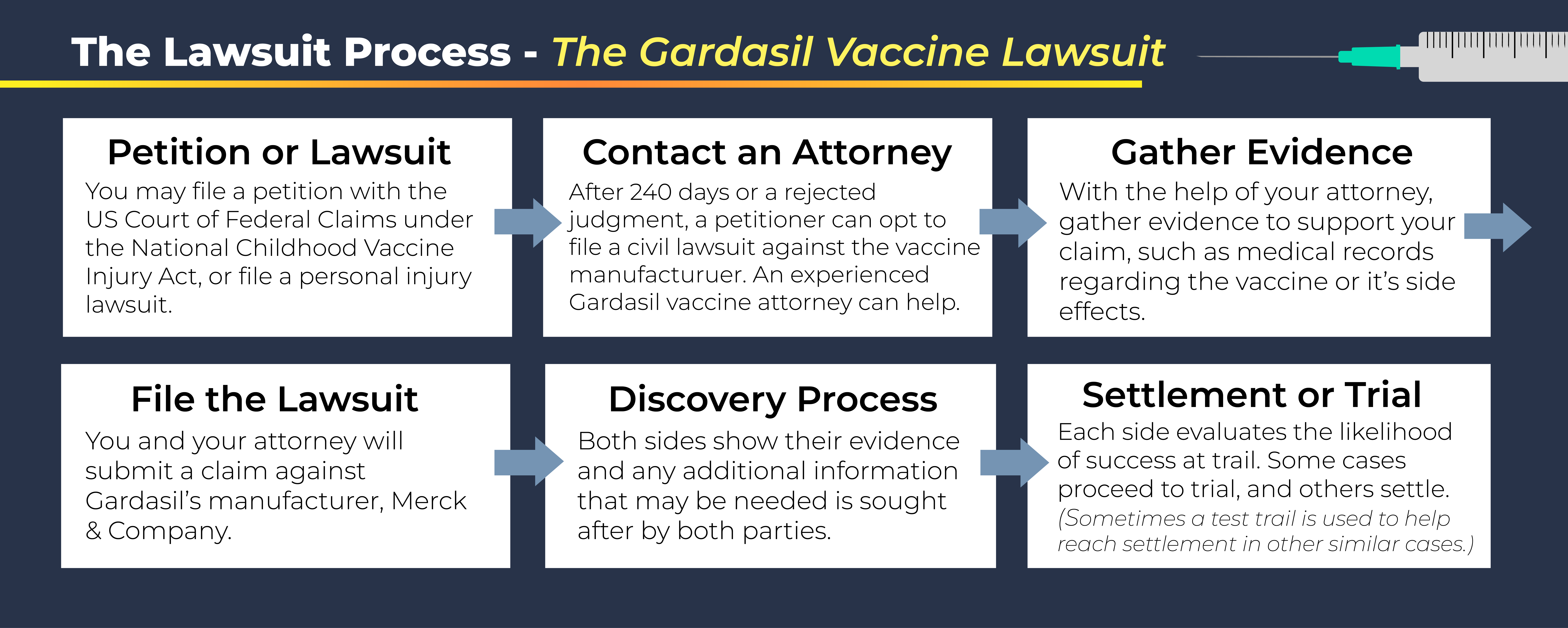
How to File a Gardasil Vaccine Lawsuit
Individuals who have suffered adverse side effects from the Gardasil vaccine may be entitled to compensation either by filing a petition with the United States Court of Federal Claims or by filing a personal injury lawsuit.
The National Childhood Vaccine Injury Act allows certain individuals who were injured as a result of a vaccine (including the HPV vaccine) to file a claim for compensation. The Act generally covers individuals who have suffered anaphylaxis, shoulder injury related to vaccine administration, and Vasovagal syncope. Only a person who received the vaccine, their parent or guardian, or the legal representative of a decedent’s estate can file a petition. After 240 days or a rejected judgment, the petitioner can opt to file a lawsuit in civil court against the vaccine manufacturer.
A Gardasil vaccine claims lawyer can help file the claim and provide guidance if the claim is rejected. Anyone experiencing side effects from the vaccine should consider filing a Gardasil claim. A lawyer can provide a free case evaluation to determine the best course of legal action based on your vaccine-related injuries.
Contact a Gardasil Lawyer
Did you suffer adverse side effects after taking the Gardasil vaccine manufactured by Merck? You may be entitled to compensation for your injuries. Depending on the circumstances of your case, you may be eligible to file a petition in the U.S. Court of Federal Claims through the National Vaccine Injury Compensation Program (VICP) or a personal injury lawsuit.
At King Law, we can provide the dedicated, experienced representation you need to make an informed decision about your case. Our Gardasil lawyers will walk you through the process, including what to do if your petition is rejected through the federal claims process. Contact our office today to schedule a free, no-obligation case evaluation.

