Complete the form below to see if you qualify for a Spinal Cord Stimulator Lawsuit

Many people who have spinal cord stimulators (SCS) implanted experience pain at the site of the battery. The battery (generator) powers the medical device. However, it can cause chronic pain that is referred to as pocket pain. Some patients who experience pain at the site of the battery need additional surgeries to reposition the battery. Other patients need to have their spinal cord stimulator removed completely.
Spinal cord stimulators have been marketed to help alleviate chronic pain. But many people have reported adverse effects related to the battery pack or implantable pulse generator (IPG) used to power the device.
These reports suggest that the IPG itself is a source of pain for patients due to charging or overheating events, premature depletion, infection, hematoma, or pressure and scar capsule irritation. Studies suggest that at least 5.5% of people experience pain at the battery site after a spinal cord stimulator implant.
This page will cover how spinal cord stimulator battery packs can cause pain, how the pain presents in patients, FDA recalls, and legal options for people who have experienced pain due to their implants.
This guide will cover the symptoms, common causes, and notable safety alerts related to spinal cord stimulators currently on the market. It will also provide advice for when to contact an attorney and what rights patients have related to SCS battery pain.
About the Spinal Cord Stimulator Battery Pain
What Is a Spinal Cord Stimulator (SCS) Battery and Why Can It Hurt?
What Are the Common Causes of Spinal Cord Stimulator Battery Pain?
What Does Spinal Cord Stimulator Battery Pain Feel Like?
Is Battery Pain Normal After Spinal Cord Stimulator Surgery?
Spinal Cord Stimulator Battery Recalls and Safety Alerts
King Law Is Investigating Spinal Cord Stimulator Battery Pain Claims
What Is a Spinal Cord Stimulator (SCS) Battery and Why Can It Hurt?
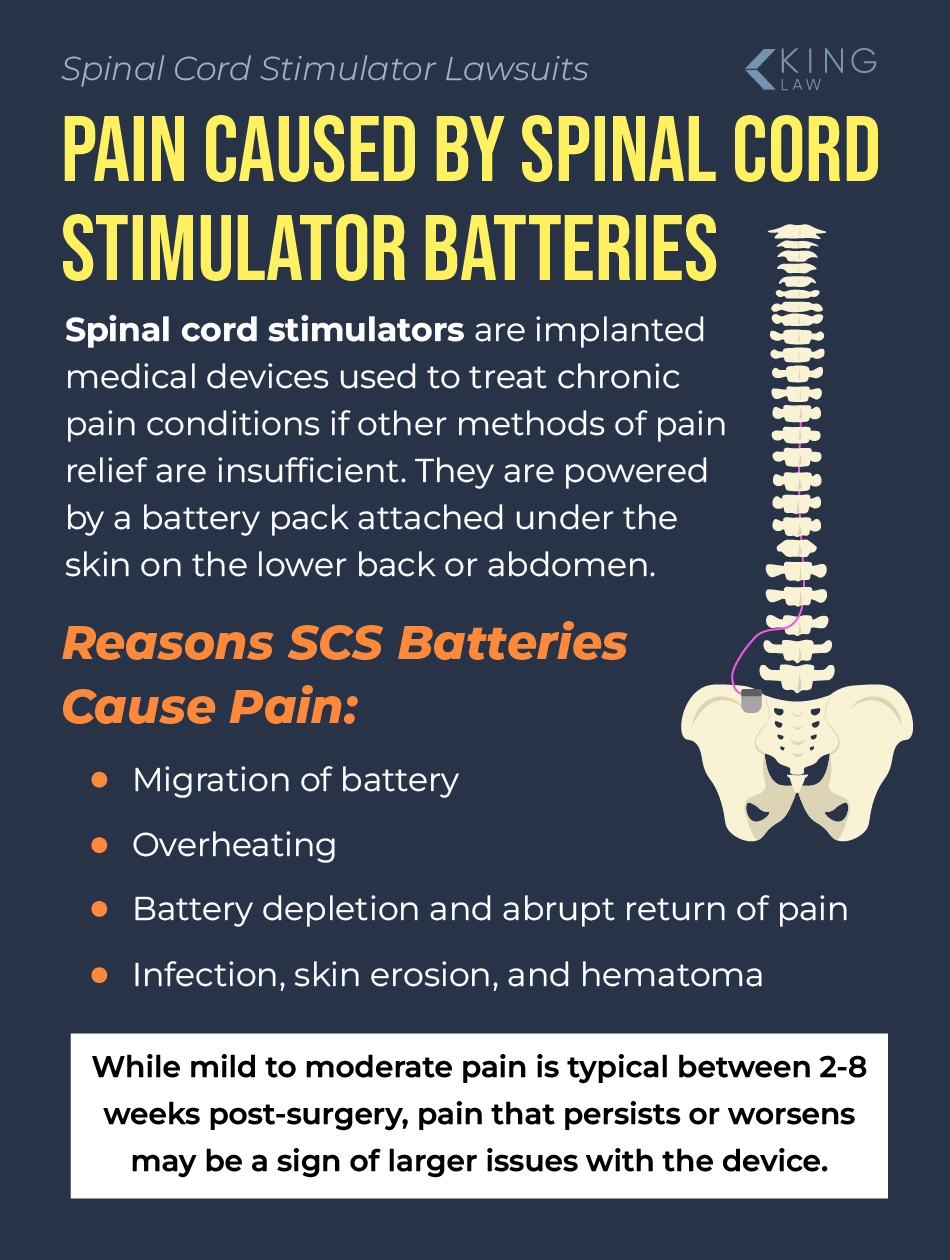
Spinal cord stimulator battery pain is discomfort or burning at the site of the implanted battery, often caused by device migration, overheating, or infection. A spinal cord stimulator battery powers the medical device. Spinal cord stimulators (SCSs) are used to treat chronic pain.
These devices consist of wires (electrodes) and a small battery pack or implantable pulse generator (IPG). The battery pack is placed subcutaneously (under the skin) near the lower back or abdomen.
In order to send these pulses, the device needs an energy source. The device uses the energy to send electrical pulses through the wires. These pulses are supposed to help lessen the pain a patient is experiencing. There are both non-rechargeable and rechargeable SCS battery systems.
Spinal Cord Stimulator Battery Problems
Spinal cord stimulator battery packs can hurt people because the packs are hard, placed under the skin, and come in contact with various parts of the body. The battery can also cause injury if it migrates or rotates, forms a pocket, or causes a scar capsule contraction. Spinal cord stimulator power packs can also cause tissue and nerve damage as a result of frequent surgeries.
SCS battery pain symptoms can include:
- Pain or burning at the implantation site
- Tingling near the device
- Redness, pain, or swelling at the battery site
- Fever of more than 101 degrees
Studies Documenting Painful Side Effects for People with Spinal Cord Stimulator Implants
According to a study published in the Journal of Patient Safety, 79% of adverse events related to spinal cord stimulators were reported as severe, and 13% were considered life-threatening. Device malfunction, migration, and infection were the primary causes of concern.
Another study, published in the journal Pain Medicine, found that 5.5% of people who had spinal cord stimulators implanted experienced pain at the device’s battery. This type of pain is referred to as SCS generator pocket pain.
What Are the Common Causes of Spinal Cord Stimulator Battery Pain?
Battery pain related to a spinal cord stimulator is a broad term used to describe several problems associated with the pain-management device. Pain at the site of the battery implant can originate from several sources.
Some of the ways spinal cord stimulator batteries cause pain include:
- Mechanical pocket pain (pain caused by the physical presence of the device)
- A heat or burning sensation during recharging
- Pain caused by battery depletion or device malfunction
- Inflammatory pain caused by a hematoma, skin erosion, or infection
- Pain caused by software or EMI failures that result in suspended therapy
Patients who experience SCS battery pain are encouraged to seek medical attention immediately. Determining the root of the problem is often critical to prompt diagnosis and treatment.
Pocket Pain, Pressure, and Scar Capsule Irritation
A study published in the Neuromodulation Journal found that 31% of patients had pain at the generator/battery site. Generator-site discomfort is a well-documented complication of spinal cord stimulators. However, the pain associated with the battery pack can become severe. The injury, often referred to as “pocket pain,” can present as pressure or a tightening around the scar capsule. It can also be the result of nerve irritation or a migration or rotation of the device.
After implantation, the device can also cause a hematoma, which is defined as a pooling of blood within the body. Management for pocket pain ranges from minor revisions to removal, depending on the severity of the pain. Both of these treatment options involve additional surgeries.
Overheating and Burning Sensation
In addition to pocket pain, people with spinal cord stimulators have reported burning at the battery/generator site. It is believed that this adverse reaction can be caused by the transcutaneous inductive charging from the rechargeable systems used in these devices. Any misalignment of the device can cause heating, resulting in a burning or stinging sensation at the implantation site.
Despite modern safeguards to prevent overheating, many people still report issues with their spinal cord stimulator’s batteries. According to research published in the Neuromodulation Journal, up to
Even patients who follow device-charging guidelines, like not charging their devices in high-temperature environments. Patients experiencing redness or warmth at the implantation site are encouraged to seek immediate attention from their healthcare provider.
Battery Depletion and Breakthrough Pain
The batteries in many spinal cord stimulators do not last as long as they are supposed to. When the batteries lose power, the patient can experience breakthrough pain.
Battery issues can shorten therapy, causing pain to return rapidly.
Many SCS devices are equipped with elective replacement indicators (ERIs), which are designed to give patients a warning when the device is nearing the end of its battery life. However, some devices have been known to have a shorter window between the ERI and end-of-service, resulting in an abrupt return of pain.
In 2024, Abbott, the manufacturer of a commonly used SCS device known as the Proclaim, issued a notice to physicians. This notice explained that the time between when the device issues an elective replacement indicator (ERI) notice and end of service can be 45-55% shorter than what was initially indicated on the label.
This means that the time between when a patient gets a warning that the battery could be replaced and when the battery dies is much shorter than indicated. When a spinal cord stimulator’s power source fails, the patient can experience debilitating pain and discomfort.
A 2023 study published in the Cochrane Library found that there was
Infection, Hematoma, or Skin Erosion at the Battery Pocket
Another problem reported by spinal cord stimulator patients was an increased risk of infection, hematoma, or skin erosion at the battery pocket. Although infections and other problems generally present early, it can also take months or even years for issues to arise.
Common signs of infection after SCS battery implantation include:
- Redness at the implantation site
- Warmth around the site
- Presence of swelling
- Drainage near the site
- Signs of fever
- Escalating pocket pain
Many individuals who suffer an adverse event related to a spinal cord stimulator battery require removal of the device or additional surgeries to repair the issue. If infection has set in, the patient is generally put on antibiotics to reduce the risk of complications.
Hematomas, or a collection of blood under the skin, can cause intense pain, swelling, and pressure. These side effects can present immediately after surgery and require a separate treatment. Some patients report a progressive skin thinning or erosion at the battery implantation. This can also cause battery pain that requires surgery to correct.
What Does Spinal Cord Stimulator Battery Pain Feel Like?
Pain caused by the battery or power source for a spinal cord stimulator can be caused by different issues. However, patients often report similar symptoms that are evidence of a serious complication. It is important to discuss any new or worsening symptoms with a healthcare provider. Even mild issues can indicate a more serious problem.
Common spinal cord stimulator battery pain symptoms:
- Burning or stinging at the IPG (battery) site
- A stabbing or sharp poking sensation
- Site is warm to the touch
- Pulling or tugging feeling
- Return of chronic pain
- Fever or chills
- Skin erosion
- Redness or drainage
- Swelling at the IPG (battery) site
Any signs of infection, such as fever or chills and discharge at the site, can indicate a severe health problem. It is imperative to seek immediate medical treatment to ensure diagnosis of the problem and prompt treatment.

Is Battery Pain Normal After Spinal Cord Stimulator Surgery?
Mild to moderate soreness at the battery pocket is common in patients, especially soon after surgery. It can take 2 to 8 weeks after implantation for tissues to heal and pain to subside. However, more severe, long-lasting pain is abnormal and can indicate a larger problem.
Pain that persists or worsens in the weeks after surgery should be investigated by a healthcare provider. Additionally, battery-site pain that limits a patient’s normal activities should be evaluated as soon as possible. Patients should talk to their doctors about any redness, discharge, or swelling at the implant site.
How Long Does Spinal Cord Stimulator Battery Pain Last?
Pain from battery packs on SCS devices can last from weeks to months. Though most patients experience pain for only a few days to a week after surgery. It can take 6 weeks to 6 months for patients to return to their previous activity levels after the implant surgery. However, new pain can emerge due to device malfunction.
As mentioned above, approximately 30% of SCS patients require removal of the device. It can take months to recover from surgery or an infection caused by the battery pack.
As always, it is important to follow the treatment plan of a healthcare provider and to report any changes in symptoms. Diagnosis often requires imaging, while treatment can entail device revision, relocation, or removal.
Spinal Cord Stimulator Battery Recalls and Safety Alerts
Several recalls and safety alerts have been issued for spinal cord stimulator devices. These recalls have been issued for a wide-range of issues, including implantable pulse generator (IPG) resets, shortened elective replacement indicator (ERI) to end of service windows, safety mode manufacturing defects, and the inability to exit MRI mode. These recalls highlight the potential safety issues associated with SCS batteries.
Notable SCS recalls and safety alerts in the US:
- Proclaim and Infinity IPGs: The FDA issued a class I recall (the most serious recall) for certain Proclaim and Infinity IPGs manufactured by Abbott. The company and regulators had received multiple reports that patients could not exit the magnetic resonance imaging (MRI) mode.
- WaveWriter Alpha: Boston Scientific issued an Urgent Medical Device Advisory for its WaveWriter Alpha Spinal Cord Stimulation IPG after receiving reports that the device could randomly and remotely reset.
- Proclaim: Abbott also issued an Urgent Medical Device Correction for certain Proclaim IPG batteries after determining the ERI to end of service window was shorter than the label indicated.
People with spinal cord stimulator implants are encouraged to consult with their medical provider to determine whether a recall or safety advisory has been issued on their device.
King Law Is Investigating Spinal Cord Stimulator Battery Pain Claims
King Law is currently reviewing claims from spinal cord stimulator patients who experienced battery-related harm, including pocket burns, device overheating, and rapid battery depletion. In many cases, these problems suspended therapy, causing the resurgence of chronic pain. Other patients had to have their SCS devices removed (which is called an explant) or repositioned, requiring additional surgeries.
Individuals who suffered harm may be able to file an SCS lawsuit related to defective design, failure to warn, negligence, or manufacturing defect. Eligibility and potential case value vary depending on the individual circumstances of the claim, such as the severity of the injury, device model, and type of harm.
Contact a Spinal Cord Stimulator Lawyer Today
Spinal cord stimulator patients who suffered battery-related harm are encouraged to seek legal counsel by contacting King Law at (585) 496-2648. Our spinal cord stimulator attorneys can help determine eligibility and will seek evidence to help substantiate valid claims against negligent manufacturers. Our team can help you understand if you qualify for a spinal cord stimulator lawsuit.
Successful claimants may be entitled to compensation for medical bills, lost wages, revision or reparative surgeries, and pain and suffering. Contact King Law today to schedule a free, confidential consultation. We only get paid if you win compensation for your case.