Lawsuits filed nationwide allege some AngioDynamics port catheters contain a defective design, which causes serious injury to patients. Lawsuits allege that AngioDynamics knew about the design defect and failed to warn consumers about the potential harms its port catheters could cause.
The injuries reportedly caused by the port catheter range from infections to blood clots and catheter fractures. Plaintiffs who experienced harm after using an AngioDynamic port catheter are seeking compensation to cover medical bills, pain and suffering, and more.
In October 2024, cases pending against AngioDynamics were consolidated into multidistrict litigation out of the Southern District of California (MDL 3125 – IN RE: AngioDynamics, Inc., and Navilyst Medical, Inc., Port Catheter Products Liability Litigation). People harmed by AngioDynamics port catheters may still be able to join this group litigation. Individuals affected by the medical device are encouraged to speak with an attorney to determine eligibility for an AngioDynamics port catheter lawsuit.
About the AngioDynamics Port Lawsuits
More than 150 lawsuits have been filed against AngioDynamics and its subsidiary, Navilyst Medical, alleging their port catheter devices cause serious harm to patients. The catheters are medical devices that are implanted in the chest area to provide vascular access and intravenously administer medication, fluids, and other therapies. The devices are reportedly designed for long-term use with no warnings about the need to be removed after a period of time.
Legal documents filed against AngioDynamics regarding their catheter ports suggest the devices contain a dangerous design defect that may cause the product to malfunction. This defect has reportedly resulted in serious injuries, including infection, blood clots, and catheter fractures. As a result, AngioDynamic port catheter patients have experienced severe harm, extraordinary medical bills, and physical deformities and report having to undergo surgery to remove or repair the device.
Lawsuits against the company further allege that they had received a number of reports about device failures and serious injuries. Despite receiving notices that the port catheters may be unsafe, AngioDynamics allegedly continued to market and sell the products. Taking legal action against the company may help some patients recover losses related to their injuries and reframe the advertising and marketing of these harmful devices.
AngioDynamics Port Catheter Lawsuits – 2025 Update
October 2, 2025: Illinois Woman Sues AngioDynamics After SmartPort Breaks Off into Heart
A woman named Janna Nicholson had the SmartPort implanted in April 2017. By August of 2017, she had to have an additional surgery to take the SmartPort fragment out of an atrium (chamber) in her heart. In 2023, she discovered an attorney advertisement saying that other people had experienced a similar issue where their SmartPort had broken and pieces migrated to other parts of their body. She is suing AngioDynamics and Navilyst for her medical expenses, lost wages, and other damages because of the company’s negligence.
September 2, 2025: AngioDynamics Claims Grow in Numbers as More People Experience Complications
There are now 218 lawsuits in the federal group claim against the AngioDynamics Smart Port catheter, which has caused many to suffer from complications like blood clots and infections. Some people have had pieces of the Smart Port break off and travel into their hearts. AngioDynamics denies all liability, but public reports and court filings suggest the medical company was aware of the risks and did not tell patients about those problems, putting many in danger. Victims who are not yet part of the growing litigation can file a claim for compensation.
August 1, 2025: Oregon Woman Files Claim Against AngioDynamics, the Defective SmartPort Maker
Another victim of the dangerous SmartPort has filed a lawsuit against AngioDynamics. Brandi Nicole Davison had the SmartPort implanted in March 2020, and she was hospitalized for an embolism (blood clot) months later. The doctors removed the SmartPort, and three years later, Brandi learned that the defective SmartPort may have been the reason she developed the blood clot. She is suing AngioDynamics for compensation for her losses.
July 2, 2025: Woman’s Estate Sues Because of AngioDynamics Port Breakage
Anna McDonald’s estate has sued AngioDynamics and Navilyst Medical after Anna experienced dangerous complications. A month after being implanted, pieces of Anna’s SmartPort broke off and moved into the right ventricle of her heart. Anna had to have emergency surgery, so her doctors could find and remove the stray pieces and the device. Many other victims had experiences similar to Anna, and they are filing individual and group lawsuits.
June 2, 2025: FDA Receives Another Report of an AngioDynamics Port Injury
Many patients have reported injuries due to their AngioDynamics port catheters. Reports are recorded in the FDA’s MAUDE (Manufacturer and User Facility Device Experience) database. In the adverse event report, a man said he developed an infection after having an AngioDynamics Smart Port CT installed. The report says the port was properly installed according to manufacturer specifications. Yet, the man experienced an infection and had to have the port removed. Infections and removal of AngioDynamics ports are two of the reasons people are filing lawsuits against the manufacturer.
May 2, 2025: AngioDynamic Port Catheter Products Liability Litigation Grows to Over 150 Cases
As of May 2, 2025, the multidistrict litigation (MDL 3125) has grown to 152 pending cases. The MDL is being heard out of the Southern District of California and presided over by U.S. District Judge Jinsook Ohta. Although the AngioDynamics port catheter lawsuit remains in the early stages of litigation, the cases continue to move forward. Centralization of the actions helps to ensure consistent rulings on matters and the streamlining of certain pretrial processes.
April 20, 2025: AngioDynamic Port Catheter Required Surgical Removal, According to Complaint
According to a legal complaint filed on April 10, 2025, a SmartPort manufactured by AngioDynamics required surgical removal after causing severe sepsis in a patient. The device reportedly degraded and failed after implantation due to the barium sulfate used during the manufacturing process. Despite being notified of prior adverse incidents regarding the SmartPort and other devices, AngioDynamics failed to warn consumers of potential safety risks. The patient, who suffered serious harm, is seeking compensation for the damages she suffered from the device.
October 3, 2024: JPML Orders Transfer of AngioDynamics Port Catheter Lawsuits to Southern District of California
The Judicial Panel on Multidistrict Litigation has ordered the transfer of 23 pending actions to the Southern District of California. The newly formed MDL (MDL 3125 – IN RE: AngioDynamics, Inc., and Navilyst Medical, Inc., Port Catheter Products Liability Litigation) is expected to grow significantly in the coming months, with the court identifying at least an additional 33 potential cases. The court found that the pending lawsuits involved common questions of fact, making them suitable for centralization (group consolidation).
February 22, 2021: AngioDynamics Issues Urgent Medical Device Recall of Smart Port-CT
On February 22, 2021, AngioDynamics issued an Urgent Medical Device Recall notice regarding their Smart Port-CT. According to the recall, over 260 kits were at risk of package failure, potentially resulting in compromised sterility. The recall was terminated on July 19, 2022. The safety of the Smart Port-CT is currently under scrutiny, as lawsuits allege the device, along with others manufactured by AngioDynamics, may have a design defect that is dangerous to patients.
January 31, 2012: AngioDynamics Announces Acquisition of Navilyst Medical
On January 31, 2012, AngioDynamics announced the acquisition of Navilyst Medical for $372 million. The acquisition helped to expand the company’s portfolio, particularly in the vascular access market. Navilyst Medical has been named in lawsuits along with AngioDynamics concerning defective port catheters that have allegedly caused serious harm to patients.
About the AngioDynamics Port Catheter Lawsuit:
Why Do People Have AngioDynamics Ports Implanted?
What Problems Have People Experienced With AngioDynamics Ports?
What Injuries Did AngioDynamics Port Catheters Cause?
What Are the Allegations in the AngioDynamics Port Catheter Lawsuits?
Did AngioDynamics Recall Their Port Catheters?
Did AngioDynamics Know Their Ports Were Hurting People?
How Do You File a Port Catheter Lawsuit Against AngioDynamics?
Who Is Eligible to File a Lawsuit Related to Port Catheter Injuries?
Port Catheter Lawsuit Estimated Settlements and Payouts
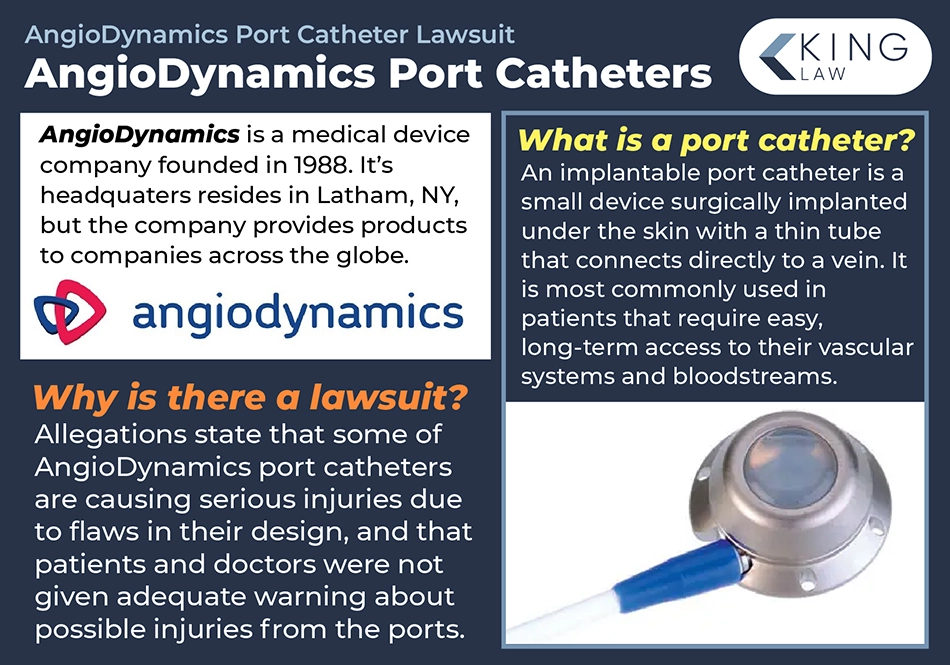
Who is AngioDynamics?
AngioDynamics is a global medical device provider that was founded in Queensbury, New York, in 1988. The company considers itself a leader in the industry, providing innovative products for vascular access and other areas. The company makes a number of power-injectable ports and other port catheters. It expanded its portfolio significantly with the 2012 acquisition of Navilyst Medical.
However, several of AngioDynamics’ port catheters have had their safety questioned as more reports of adverse events are filed. Allegations include that the company knew its products contained a dangerous design defect and that it failed to warn consumers about the potential for harm. Over 150 cases have been consolidated into an MDL pending out of the Southern District of California. The litigation is expected to grow as additional victims come forward.
Why Do People Have AngioDynamics Ports Implanted?
There are several reasons why people may have had AngioDynamics ports implanted. The port devices are widely used to provide an infusion of medications, chemotherapy, IV fluids, and other therapies. The implantable ports, such as the SmartPort, are designed for patients who require long-term access to their vascular system or bloodstream.
Implanting a port catheter can help alleviate the need for regular blood draws and may make the delivery of medication easier for the patient. Cancer patients frequently use port catheters to help administer chemotherapy. These catheter ports contain several parts:
- Injection port reservoir: Connected to a catheter tube implanted just beneath the skin.
- Catheter tube: Small, thin tube connected to a large vein that acts as a conduit for IV therapies, fluids, or medications.
- Septum: Serves as the access point.
How Are Port Catheters Implanted?
Implanting a port catheter requires a surgical procedure, generally done as an outpatient procedure. While the patient is under general or local anesthesia, the port (a small, round medical device) is implanted beneath the skin and connected to a vein. The port is connected to a catheter which serves as a conduit for the fluid, medication, or other therapy to be administered.
For patients who require regular blood draws or long-term treatments, a port can provide a convenient and less painful option compared to consistent injections or withdrawals. While the port is implanted, it must be maintained and cleaned regularly. Signs of infection or newly developed adverse symptoms should be reported to a healthcare provider as quickly as possible.
What Problems Have People Experienced With AngioDynamics Ports?
Port catheters can cause many problems for patients. Lawsuits filed against AngioDynamics allege that the design of its catheter ports may cause serious injury to patients. Prior to the current litigation, the company was put on notice about potential injuries caused by their products. Healthcare providers had filed a large number of adverse event reports (AERs) about the ports, using the FDA’s reporting system.
Complications due to a catheter port failure:
- Hemorrhaging
- Infection (including sepsis)
- Blood clots (vascular occlusion or thrombosis)
- Fever
- Pain
- Migration or fracture of the device requiring surgery
- Tissue or organ perforation
The catheters are designed for long-term use, however, a design defect can cause the port to fail or degrade. In some instances, the catheter fractures or migrates, causing a blockage and requiring surgery to repair or remove the port or its fragments.
One of the potential defects in the catheter ports relates to the connection sleeve or where the catheter is joined to the body of the injection port. The defect may cause the catheter, or thin tube, to separate from the port body.
For cancer patients, receiving chemotherapy drugs, the separation may cause the drugs to come into contact with tissues. Known as extravasation, the leakage of these cytotoxic chemotherapy drugs can cause exceptional harm, such as tissue necrosis, debilitating pain, and long-term treatment.
Design Defects Named in Port Catheter Lawsuits
Legal action taken against AngioDynamics in relation to its port catheters alleges that the medical devices contain multiple design defects. One of the defects is that the connection sleeve was improperly designed. In particular, it is believed that there may be a mismatch between the diameter of the sleeve and the catheter tube and port. The ill-fitting sleeve can cause the tube to separate from the port. If the separation occurs while the device is implanted beneath the skin it can cause the medication to leak into the body or the device to fracture or migrate.
AngioDynamics Products Listed in the Medical Port Lawsuit
Multiple AngioDynamics products have been the subject of lawsuits filed in federal and state courts. In particular, certain models of the company’s LifePorts and SmartPorts have come under scrutiny. Individuals who have had these implanted and suffered an adverse health effect are encouraged to seek legal counsel to determine their eligibility for filing a lawsuit.
Devices named in the AngioDynamics Port Catheter Defect Lawsuit:
- Vortex Port Catheter
- BioFlo Port Catheter
- SmartPort CT Catheter
- Xcela Plus Port Catheter
- Navilyst PowerPort
What Injuries Did AngioDynamics Port Catheters Cause?
Injuries resulting from a port catheter can range in severity. Unfortunately, problems related to port catheters are not unusual. As many as 1 out of 3 port patients experience issues, with infection being the most common issue. Some infections caused by a port catheter can be fatal. Any new or worsening symptoms experienced after implantation should immediately be reported to a healthcare provider.
Risks associated with port catheters:
- Infection: Infections following the implantation of a port catheter are common. Some infections can be life-threatening, such as sepsis.
- Catheter fracture or migration: Defects in the port catheter can cause the device to break or migrate. When the device or fragments of the device move out of place, they can damage tissues or organs, or even cause an embolism.
- Thrombosis (blood clots): Port catheter patients have reported developing blood clots. Blood clots can increase a person’s risk of serious health problems, such as a stroke.
- Damage to blood vessels: A defective catheter may fracture or break, causing vascular damage.
- Organ and tissue damage: Catheters have also been known to cause damage to internal organs due to port migration or fracture.
- Death: In rare cases, complications related to a defective port may result in death.
In addition to these injuries, some patients may require surgical intervention to repair damage caused by the port catheter or to remove the device.
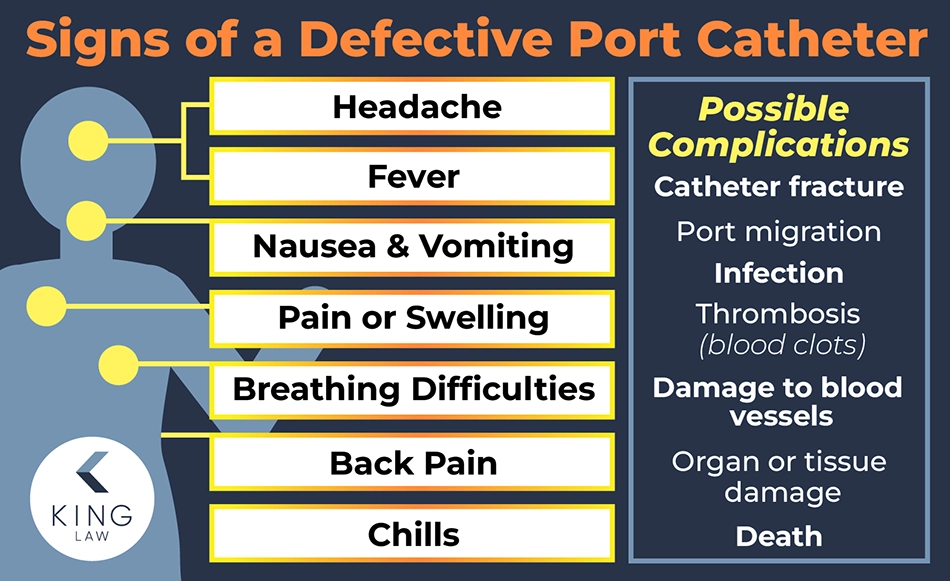
Signs and Symptoms of Port Catheter Defects
A defective port catheter can cause severe mental and physical harm to a patient. The device may migrate or fracture, causing damage to nearby organs, or it may result in serious, potentially deadly infections. Any new or worsening symptoms experienced after implantation should be immediately reported to a healthcare provider. Left untreated, complications related to a defective port catheter can cause permanent damage.
Signs and Symptoms of a Port Catheter Defect:
- Fever
- Chills
- Nausea and vomiting
- Back pain
- Headache
- Difficulty breathing
- Pain or swelling
How Do Doctors Treat Patients Whose Ports Failed?
The treatment for a failed AngioDynamics port catheter differs significantly depending on the specific injury. While some minor infections may resolve with antibiotics or relatively little intervention, other injuries may require substantial treatment. In some instances, such as if a device has fractured, the patient may have to be hospitalized or even undergo surgery to remove the port. It is important to discuss any and all symptoms with a healthcare provider to ensure prompt diagnosis and treatment.
Difficulties Removing AngioDynamics Ports
According to reports, AngioDynamics port catheters can be challenging to remove, particularly if the device contains a defect. Common defects in the products may result in degradation or fracture. In cases where the device has broken apart into small pieces, patients have had to endure complicated and often repeat heart surgeries. While surgical intervention can help, it is not always successful at completely removing all of the parts of the port catheter.
What Are the Allegations in the AngioDynamics Port Catheter Lawsuits?
Lawsuits filed against AngioDynamics allege the violation of a number of legal principles, from negligence to product liability. Plaintiffs suggest that the medical device manufacturer knew or should have known about the potential dangers associated with the use of its product and failed to warn consumers.
Lawsuits filed as part of the AngioDynamics Port Catheter MDL include allegations of:
- Breach of express warranty: Allegations against the company include that it breached the express warranty by producing packaging, advertising, and marketing materials stating the products were safe.
- Breach of implied warranty: Patients who suffered harm as a result of a defective AngioDynamic port catheter allege the company breached an implied warranty by putting a reportedly unsafe product on the market.
- Design defect: Legal documents accuse AngioDynamics of manufacturing a product with a defective design that can cause harm even when implanted properly.
- Failure to warn: Complaints filed against AngioDynamics allege the company failed to warn consumers about the dangers associated with port catheter use.
- Fraudulent concealment: Allegations suggest AngioDynamics continued to market the products as safe despite knowing the risk of harm.
- Negligence: Legal documents allege the company was negligent in the design, testing, manufacturing, and marketing of the port catheters.
- Violation of state consumer protection laws: Complaints also accuse the company of violating state consumer protection laws, including deceptive and unfair trade practices.
How AngioDynamics Failed to Protect Consumers
Multiple lawsuits filed against AngioDynamics accuse the company of failing to protect consumers by knowingly manufacturing and marketing a product that it knew to be potentially unsafe. Furthermore, allegations include that the company failed to warn consumers about the potential for harm after receiving large quantities of adverse health reports.
Accusations against AngioDynamics related to Defective Port Catheters:
- AngioDynamics failed to establish a removal procedure that was safe, particularly in the event of failure leading to complications.
- AngioDynamics marketed its port catheters as safe, effective, and minimally invasive to healthcare providers and patients.
- The company marketed the devices as a safer and more effective option compared to other products and procedures.
- AngioDynamics knew that the device contained a design defect but failed to correct it or warn consumers, causing an unreasonable risk of harm to patients.
- The company failed to adequately test the ports in question prior to bringing them to market, causing an unsafe medical device to be sold.
- AngioDynamics failed to provide adequate safety information or provided incomplete or misleading information to healthcare providers about the safety of the ports, including the SmartPort.
Did AngioDynamics Recall Their Port Catheters?
Prior to the current lawsuits, AngioDynamics has issued at least two recalls of their ports. While these recalls remain unrelated to the ongoing litigation, they underscore the potential safety problems associated with these devices.
Recall of SmartPort CT
On February 22, 2021, AngioDynamics issued an Urgent Medical Device Recall notice for a batch of its SmartPort CT implantable infusion port kits. According to the recall, the package integrity of the 267 kits may have been susceptible to failure, resulting in compromised sterility of the device. The recall was terminated on July 19, 2022.
Recall of Vortex MP Port
On September 20, 2022, AngioDynamics issued an Urgent Medical Device Correction Letter for a small batch of its Vortex MP Port Titanium Low Profile Implanted Port. The recall was initiated as a result of printed instructions not being provided with the units. Failure to follow proper instructions may result in harm to the patient during implantation.
Did AngioDynamics Know Their Ports Were Hurting People?
In addition to the aforementioned recalls, AngioDynamics had received many adverse event reports (AERs) from doctors and patients regarding their devices. According to these reports, the devices were causing serious harm, including hemorrhaging, blood clots, and infections. There were also a growing number of reports that the device was prone to failure after a period of time, resulting in catheter fracture or migration.
Despite these concerns, AngioDynamics continued to manufacture and market the ports in question. No subsequent warnings were provided about the potential for harm associated with using the devices.
Adverse Event Reporting to the FDA
One of the key indications that AngioDynamics knew about the potential dangers associated with the use of its port catheters is the number of Adverse Event Reports received through the FDA Adverse Event Reporting System (FAERS). The FAERS is a public dashboard hosted by the U.S. Food and Drug Administration (FDA), where patients and providers can submit an undesirable experience with a drug or medical device.
Adverse Events reported to the FDA regarding AngioDynamics Port Catheters:
- Hemorrhage (bleeding from a damaged blood vessel)
- Cardiac/pericardial tamponade (Accumulation of fluid or blood in the pericardial space)
- Cardiac arrhythmia (abnormal heart rate)
- Severe and persistent pain
- Tissue or organ perforation (tears in tissues or organs)
- Death
Adverse Event Report on the Vortex TR Single Titanium Port System
According to one Adverse Event Report about an AngioDynamic port, a plaintiff experienced life-threatening injuries after his Vortex TR Single Titanium Port System malfunctioned. The port was initially implanted to administer chemotherapy drugs to the patient. After implantation, the patient began experiencing extreme pain, which was determined to be caused by the port. During removal, it was discovered that a piece of the catheter had broken off and migrated to the patient’s heart.
A second emergency surgery was required to remove the fragment from the heart. The injury reported caused the plaintiff to sustain significant physical and mental pain and suffering and a permanent deformity.
How Do You File a Port Catheter Lawsuit Against AngioDynamics?
Individuals who have experienced severe side effects from an AngioDynamics port catheter are encouraged to seek the support of a qualified attorney. Depending on the situation, eligible patients may be entitled to compensation for their injuries, including recovery related to medical expenses, lost wages, pain and suffering, and more. It is important to act quickly, as there may only be a limited amount of time to take legal action.
Steps to file an AngioDynamics Port Catheter lawsuit:
- Contact an experienced attorney: The first step in filing a lawsuit against AngioDynamics is to consult with an experienced attorney. An attorney can help determine whether all eligibility requirements are met.
- Gather the necessary evidence: Prior to filing, plaintiffs must gather all evidence necessary to support their claims. Evidence that may prove critical to a port catheter defect case includes medical records, proof of out-of-pocket losses, hospitalization documents, specialist referrals, and treatment recommendations.
- Review the claim for legal requirements: All cases are subject to strict legal requirements, including a statute of limitations or deadline within which a case must be filed. An attorney can help review the claim to ensure all legal requirements are met and that the best course of action is taken.
Who Can Join the Multidistrict Litigation Against AngioDynamic and Navilyst?
In October 2024, the Judicial Panel on Multidistrict Litigation issued an order transferring nearly two dozen cases to an MDL out of the Southern District of California. The consolidation of these cases into multidistrict litigation helps to ensure consistent rulings and streamline the pretrial process.
Centralization of cases can help to ensure complex litigations, such as defective device claims, are handled efficiently. Individuals who have experienced harm (such as organ or tissue perforation, infections, or blood clots) after having an AngioDynamics port implanted may be able to join the current MDL and are encouraged to seek legal representation.
Statute of Limitations for AngioDynamics Medical Port Lawsuits
Product liability and personal injury claims, such as those filed against AngioDynamics and Navilyst Medical for their defective port catheters, are subject to a statute of limitations. A statute of limitations is a legal deadline within which a claim must be filed. Failure to file within the statute of limitations may result in the claim for compensation being denied.
Statutes of limitations are state-specific. In most cases, states give plaintiffs between 1 and 3 years from the date of injury or the date the injury is discovered to file a claim. Due to the complexities of legal deadlines and statutes of limitation, it is strongly recommended that individuals consult with a qualified attorney as early as possible.

Can You File a Lawsuit if You Recently Discovered Your Injuries Were Due To AngioDynamics’ Negligence?
Patients who have recently discovered injuries related to the implantation of an AngioDynamics port catheter may be eligible to take legal action. However, it is important to discuss your case with an experienced attorney as soon as possible to discuss legal options.
It is essential to discuss any new or worsening symptoms experienced after implantation with your healthcare provider. In some cases, patients were unaware that symptoms were related to their port catheter or that it was the result of a defective design. Complications resulting from a defective device may result in compensation but should be diagnosed promptly. In some instances, even if the statute of limitations appears to have expired, individuals may still be able to file a lawsuit.
Who Is Eligible to File a Lawsuit Related to Port Catheter Injuries?
Individuals who suffered complications or injuries after having an AngioDynamics (or Navilyst Medical) port catheter implanted may be eligible to take legal action against the manufacturer. Patients must have medical records indicating the type of port implanted and the injuries associated with the device. Common injuries experienced by potential plaintiffs include blood clots, infection, and device fracture or migration.
Eligible injuries related to an AngioDynamics port catheter defect lawsuit:
- Bloodstream infection or sepsis
- Cardiac arrhythmia
- Cardiac or pericardial tamponade
- Device-related organ damage
- Deep vein thrombosis (DVT) or blood clots
- Embolization of catheter
- Hematoma
- Hemorrhaging or bleeding
- Infection
- Necrosis (dead tissue) around port site
- Perforations of tissues, vessels, and organs
- Pulmonary embolism
What Information Will You Need to File an AngioDynamics Port Lawsuit?
To file a successful AngioDynamics port catheter lawsuit, individuals must gather evidence to support their claims. Necessary documents in a defective port case may include medical records, employment records, and statements from doctors or specialists regarding the complications experienced after implantation.
Evidence needed in an AngioDynamics port lawsuit:
- Medical records: Medical records indicating the type of port installed, date of surgery, diagnosis of complications, and subsequent intervention often prove critical in product liability claims against medical device manufacturers.
- Employment records: Employment records showing time off work related to the injury may help to substantiate your claim and lead to a larger recovery, including compensation for lost wages.
- Statements from medical professionals: Statements from medical professionals about the cause of your injuries or the dangers of the device.
- Financial records: Financial statements indicating out-of-pocket losses related to AngioDynamic port catheter complications.
Port Catheter Lawsuit Estimated Settlements and Payouts
Settlements in an AngioDynamic port catheter defect lawsuit are expected to range between $50,000 and $300,000 depending on the individual circumstances of the case. Factors that may influence payout amounts include the severity of the injury, long-term side effects, whether it resulted in a deformity or disability, and the impact on a person’s quality of life. Compensation from a port catheter lawsuit can help to cover injury-related damages such as present and future medical expenses, lost wages, loss of future earning capacity, pain and suffering, and more. Family members may be able to pursue a lawsuit if someone passed away from injuries related to their AngioDynamics port.
Contact A Lawyer About Your Port Catheter Injuries
Individuals who have suffered harm after having an AngioDynamics port catheter implanted are encouraged to contact King Law to schedule a consultation. Initial case evaluations are complementary and can be scheduled by calling (585) 684-7233 or submitting an online form. A member of King Law’s intake team will contact you as quickly as possible.
How King Law Supports Those Harmed by Port Catheters
King Law is well-versed in handling mass tort litigation and product liability cases. Our firm is currently accepting AngioDynamics port catheter lawsuits and will work tirelessly to ensure individuals affected by these devices receive the compensation and justice they deserve.
Reasons to contact King Law:
- Cases are accepted on a contingency fee basis, meaning there are no legal fees unless the client wins
- Client-focused representation
- True access to legal team
- Years of experience
At King Law, we understand that this may be a difficult time for you and your family. We are here to help. In many cases, these potentially defective medical devices have deeply impacted a person’s quality of life. You deserve a legal team that will fight for you.