
Patients who took Dupixent are filing lawsuits because they developed a type of cancer called cutaneous T-cell lymphoma (CTCL). Studies support a connection between Dupixent and cutaneous T-cell lymphoma diagnoses. Patients believe they should have been warned about this increased risk. Our team of attorneys is investigating cases from people who took Dupixent (dupilumab) for their dermatitis (eczema), COPD, asthma, or other conditions and then developed CTCL.
On this page, you will learn what the Dupixent lawsuit is about, how Dupixent and cutaneous T-cell lymphoma are connected, and the risk to Dupixent patients. You will also find studies that link Dupixent usage to cutaneous T-cell lymphoma. This article also provides information on who can file a Dupixent lawsuit, what compensation they may be entitled to, and the deadlines they must follow to preserve their legal rights.
About the Dupixent Lawsuit
What Is the Dupixent Lawsuit About?
What Is Dupixent (Dupilumab) and How Does It Work?
What Is the Connection Between Dupixent and T-Cell Lymphoma (CTCL)?
What Is the Dupixent and Lymphoma Risk?
Studies Link Dupixent Use to T-Cell Lymphoma in Patients
Who Can File a Dupixent Lawsuit?
What Damages Can I Recover in a Dupixent Lawsuit?
Is There a Deadline to File a Dupixent Lawsuit?
What Are the Estimated Settlement Amounts in a Dupixent Lawsuit?
King Law Is Investigating Dupixent and T-Cell Lymphoma Claims
What Is the Dupixent Lawsuit About?
Here are the key points of the Dupixent lawsuit:
- One of the side effects of Dupixent (Dupilumab) is that it likely increases a person’s risk of cutaneous T-cell lymphoma (CTCL), a type of non-Hodgkin lymphoma.
- The FDA is evaluating the need for potential regulatory action against Dupixent.
- People are suing Dupixent’s manufacturers (i.e., Sanofi, Regeneron Genzyme, and Regeneron) for failure to warn patients of the increased CTCL risk and for using misleading marketing techniques.
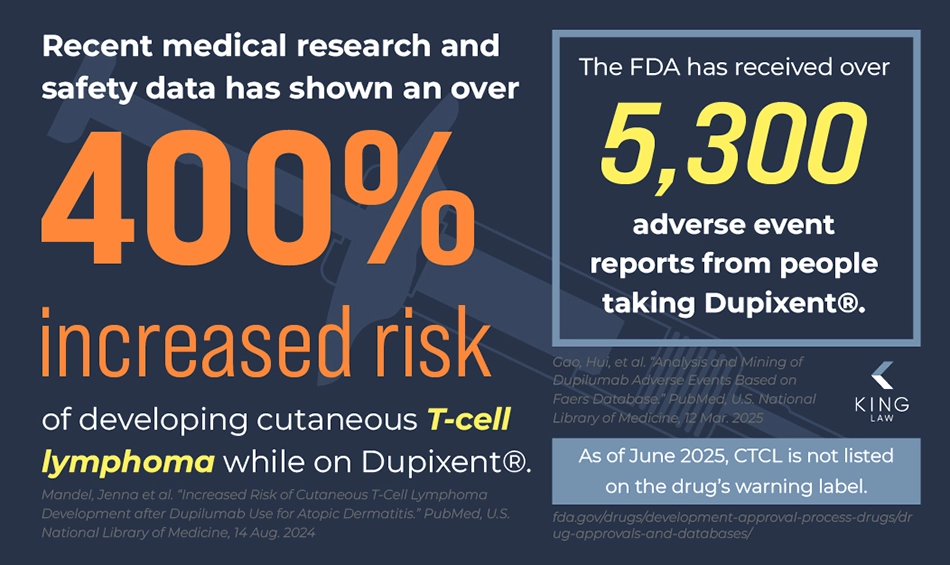
- The FDA has received more than 5,335 reports of adverse reactions from people taking Dupixent (according to the US FDA Adverse Event Reporting System (FAERS)).
- Dupixent’s makers do not currently list CTCL as a side effect on the drug’s warning label.
- People who developed this type of lymphoma are seeking settlements and compensation for their injuries from Dupixent.
- There is no class action at this time. But, a type of federal group lawsuit could be created for people harmed by Dupixent (a multidistrict litigation)
Patients are filing lawsuits because they were prescribed Dupixent for chronic conditions and then developed cutaneous T-cell lymphoma. They believe Dupixent increased their chances of developing this rare form of non-Hodgkin lymphoma. FDA adverse event reporting systems show a concerning link between Dupixent and cutaneous T-cell lymphoma. However, Dupixent’s manufacturers did not include this warning on the medication’s label.
Patients also believe that Dupixent’s makers were negligent in how they marketed the medication, and they think they may have downplayed the potential complications from taking it. Cutaneous T-cell lymphoma can look like atopic dermatitis (eczema) in its early stages, so patients also believe that the way Dupixent was marketed may have delayed many patients’ cancer diagnoses.
Is the Dupixent Lawsuit a Class Action Lawsuit?
At this time, Dupixent lawsuits are not class actions. Instead, patients and surviving loved ones are filing individual civil lawsuits in state and federal courts. Because each patient’s injuries and losses may be so different from each other, it is less likely that there will be a Dupixent class action. However, as more Dupixent patients file lawsuits, these cases may be consolidated into a multidistrict litigation (a type of federal group claim). The MDL structure allows each patient to have their case heard individually, and multiple patients can pool resources to strengthen their cases and streamline the litigation process.
Dupixent (Dupilumab) 2025 Updates
June 2025: FDA Approves Dupixent to Treat Painful Skin Condition Called BP
Despite rising safety concerns, the FDA approved Dupixent for the treatment of bullous pemphigoid (BP) on June 20, 2025. At the time, it is the only targeted treatment available to BP patients in the U.S. The disease causes large fluid-filled blisters to appear on the skin, often near creases in the skin.
May 2025: Study Finds Dupixent Has 30 Times the Reporting Rate of the Average Drug
An analysis published in the Journal of Allergy and Clinical Immunology examined FAERS data for Dupixent. FAERS is a reporting system run by the FDA. Doctors and patients use it to report adverse reactions to drugs and medical devices. According to the study, people taking Dupilumab reported adverse events at more than 60 times the rate of the average drug reporting rate. Between January 2017 and the fourth quarter of 2023, there were 181,575 unique reports of adverse events (AEs) associated with Dupixent. Of these, 606 were for neoplasms (cancers).
April 2025: FDA Approves Dupixent to Treat Skin Condition Called CSU
On April 18, 2025, the FDA approved Dupixent to treat chronic spontaneous urticaria (CSU), the first new treatment for the disease in more than 10 years. CSU is a condition where a person develops chronic hives, with episodes often lasting for 6 weeks or more.
September 2024: FDA Approves Dupixent to Treat COPD
On September 27, 2024, the FDA approved Dupixent to treat certain cases of chronic obstructive pulmonary disease (COPD). The drug is approved as an add-on treatment for adults whose COPD is not adequately controlled and of the eosinophilic phenotype (a subtype of COPD). Dupixent is the first biologic medicine receiving FDA approval for people with COPD.
August 2024: Large Study Finds Atopic Dermatitis Patients Using Dupixent 4.5 Times More Likely to Develop Lymphoma
A study published in the Dermatologic Therapy Retrospective Review looked at two groups of 19,612 patients with atopic dermatitis. The group taking Dupilumab was 4.59 times more likely to be diagnosed with cutaneous T-cell lymphoma than the group not taking the drug. About 62% of patients were diagnosed within one year of taking the drug. About 46% of patients diagnosed were under 60 years old.
August 2024: Study Says People Who Use Dupixent for Atopic Dermatitis (Eczema) Four Times More Likely to Get Lymphoma
A retrospective study, published in the Journal of American Academy of Dermatology found that patients who received Dupixent injections for eczema were 4.1 times more likely to be diagnosed with cutaneous T-cell lymphoma (CTCL), even after they stopped using the drug. Of the 41 patients diagnosed with CTCL, 27 had been on the drug for more than 1 year.
May 2022: FDA Approves Dupixent as A Treatment for EOE
Another approval is granted to Sanofi and Regeneron for their biologic drug Dupixent. Dupilumab is granted approval to treat eosinophilic esophagitis (EOE) as a first-line treatment for people ages 12 and older. The FDA would grant approval for the drug to be used in children ages 1 and older, as the first and only treatment for children with EOE.
June 2019: FDA Approves Dupixent for Treatment of Chronic Nasal Polyps
On June 26, 2019, the FDA gives their approval for Dupixent to be used as a treatment for chronic rhinosinusitis with nasal polyposis (CRSwNP). This approval is given for adults whose disease is not controlled through other treatments or medications. On September 13, 2024, the FDA would approve Dupixent as the first and only treatment for adolescents with CRSwNP.
March 2019: FDA Approves Dupixent As Treatment for Atopic Dermatitis in Adolescents
The FDA gives approval for adolescents to receive Dupixent injections to treat their moderate-to-severe atopic dermatitis and eczema. This means the drug is approved for administration in patients 12 to 17 years old. The FDA would later grant approval for use in patients 6 to 11 years old. This second approval came in May of 2020.
October 2018: FDA Gives Second Approval to Dupixent for Treating Asthma
On October 19, 2018, Sanofi announced that the FDA has approved Dupixent to treat certain types of asthma. The approval is given to Dupixent as an add-on maintenance therapy in patients with moderate-to-severe asthma. It is approved for ages 12 and up and for people with eosinophilic phenotype or oral corticosteroid-dependent asthma. The FDA would later grant approval for the drug to be used in children ages 6 to 11 who have moderate-to-severe asthma.
March 2017: FDA Approves Biologic Drug Called Dupixent (Dupilumab) to Treat Eczema and Atopic Dermatitis (AD)
On March 28, 2018, Regeneron and Sanofi announced the FDA’s approval of their new drug, Dupixent. The initial approval is given for treating moderate-to-severe atopic dermatitis (the most common form of eczema) in adults. It is the targeted biologic therapy for adults with the condition, which is a chronic inflammatory disease of the skin. It can cause debilitating pain and itching. At the time of Dupixent’s approval, Regeneron says it is estimated that 300,000 are in need of new treatment options for their atopic dermatitis (eczema).
What Is Dupixent (Dupilumab) and How Does It Work?
Dupixent (dupilumab) is a prescription drug called a biologic medication. It is given as an injection to treat various skin and respiratory conditions. It is as an interleukin inhibitor, which means it blocks certain proteins that promote inflammation. It belongs to a class of drugs called monoclonal antibodies. It was originally approved by the FDA in 2017 to treat eczema, but has received approval for other conditions, including asthma, in 2018.
The FDA has approved Dupixent to treat the following eight conditions:
- Moderate to severe eczema: Approved for moderate to severe chronic atopic dermatitis
- Asthma: Approved as an add-on maintenance treatment for uncontrolled moderate-to-severe eosinophilic or oral steroid-dependent asthma
- Chronic obstructive pulmonary disease (COPD): Approved as an additional treatment for inadequately controlled COPD with high blood eosinophils
- Chronic rhinosinusitis with nasal polyps (CRSwNP): Approved as an add-on maintenance treatment for uncontrolled nasal polyps
- Eosinophilic esophagitis (EOE): Approved to treat an inflamed esophagus caused by too many white blood cells
- Chronic spontaneous urticaria (CSU): Approved for treatment of hives not controlled by H1 antihistamines
- Bullous pemphigoid (BP): Approved to treat a skin condition that treats itchy, fluid-filled blisters
- Prurigo nodularis (PN): Approved to treat a skin condition that causes severe itching
Dupixent changes how your immune system works, with the goal of preventing your body from reacting to substances that are not an actual threat. Specifically, Dupixent turns off the IL-4 and IL-13 protein immune pathways. These proteins tell your body to become inflamed in response to attacks on your immune system. However, in people with conditions like eczema or asthma, the body has an inflammatory response to triggers that are not threats.
Dupixent helps calm down the skin irritation, redness, lesions, and inflammation that people experience when they have eczema that other medications have not helped. Dupixent is also offered to patients with treatment-resistant chronic obstructive pulmonary disease (COPD) or asthma.
While many people have experienced relief when taking Dupixent, safety data show that Dupixent may cause, reveal, or speed up cutaneous T-cell lymphoma in patients. Because of this, patients are filing legal actions to hold the manufacturers accountable for not disclosing these risks.

What Is the Connection Between Dupixent and T-Cell Lymphoma (CTCL)?
Dupixent suppresses certain inflammatory pathways, which is why the drug can be a useful treatment for certain skin and lung conditions. However, this suppression may make it more difficult for the body to detect abnormal T-cell growth and T-cell lymphoma.
Scientists are still trying to figure out if Dupixent causes, increases the risk of, or unmasks cutaneous T-cell lymphoma. Many researchers believe that Dupixent can reveal or trigger pre-existing cutaneous T-cell lymphoma rather than causing it directly. However, four-fold diagnosis rate of CTCL among Dupixent patients has warranted continued research that could benefit patients.
What Is the Dupixent and Lymphoma Risk?
Medical research and safety data show an association between Dupixent (dupilumab) use and cutaneous T-cell lymphoma (CTCL). For example, one scientific study found that patients using Dupixent are 4 times more likely to develop cutaneous T-cell lymphoma.
Cutaneous T-cell lymphoma is a rare type of cancer, affecting 8.55 out of 1 million people between 2000 and 2018. However, one study has found that Dupixent patients who are taking the medication for atopic dermatitis are 4.59 times more likely to be diagnosed with CTCL than those who have atopic dermatitis and are not on Dupixent.
The data suggest that adult patients are more likely to be diagnosed with cutaneous T-cell lymphoma within their first year after starting Dupixent. All of the eczema patients in the control group were over 60 when diagnosed with cutaneous T-cell lymphoma. But, only half of the Dupixent eczema patients were 60 or older when diagnosed with cutaneous T-cell lymphoma. These data support that taking Dupixent may lead to CTCL at a younger age.
FDA Alert for Patients Taking Dupixent (Dupilumab)
As of 2025, Dupixent (dupilumab) is listed on the FDA’s list of Potential Signals of Serious Risks/New Safety Information Identified by the FDA Adverse Event Reporting System (FAERS). According to reports, the agency is investigating the risk level of developing cutaneous T-cell lymphoma after receiving Dupixent injections. The FDA is evaluating the need for regulatory action.
Studies Link Dupixent Use to T-Cell Lymphoma in Patients
A growing body of research shows a connection between Dupixent use and cutaneous T-cell lymphoma in patients with atopic dermatitis (eczema), COPD, and asthma. These studies show a marked increase in cutaneous T-cell lymphoma rates among Dupixent users compared to the general population and those with eczema who have not taken Dupixent. Scientists are working hard to determine what is driving up the cutaneous T-cell lymphoma rates.
Study Highlight: Dupixent and the Risk of Cutaneous T-Cell Lymphoma
Study authors and publish year: Hasan et al., 2024
Study summary: Researchers studied a group of people who were diagnosed with atopic dermatitis (eczema) and prescribed Dupixent. The researchers discovered that those who took Dupixent for their eczema were four times more likely to develop cutaneous T-cell lymphoma than those who had eczema and were not given Dupixent. In this study, most of the cutaneous T-cell lymphoma diagnoses occurred after the patients had been taking Dupixent for at least a year.
The researchers noted that the study tracked an increased risk of Dupixent patients being diagnosed with cutaneous T-cell lymphoma, but they did not look into whether Dupixent causes this type of cancer. Additionally, since they used data from a database, the researchers said misclassifications might be included in their sample. However, the findings add to a body of research showing similar associations between Dupixent and cutaneous T-cell lymphoma, and additional investigation is warranted.
Study Highlight: Adverse Events and Severe Side Effects Reported to FDA
Study name: Analysis and mining of Dupilumab adverse events based on FAERS database (FAERS Disproportionality Analysis)
Study authors and publish year: Gao et al., 2025
Study summary: A study of FDA adverse event data showed a strong connection between Dupixent and cutaneous T-cell lymphoma. Although the rates of cutaneous T-cell lymphoma were low, the researchers noted that it could be a new type of adverse event being reported. The authors encouraged future research into how Dupixent interacts with the immune system and may increase the risk of cutaneous T-cell lymphoma.
Study Highlight: The Relationship Between Dupilumab and CTCL
Study name: Case Report: Cutaneous T-cell lymphoma associated with biologic therapy: three cases and a literature review (Case Series & Literature Review)
Study authors and publish year: Li et al., 2025
Study summary: Researchers examined the relationship between Dupixent use and cutaneous T-cell lymphoma diagnoses. The researchers discovered that 27 out of 31 patients with atopic dermatitis who were given Dupixent (dupilumab) developed a form of cutaneous T-cell lymphoma thereafter.
The authors noted that atopic dermatitis and early forms of cutaneous T-cell carcinoma look very similar in their early stages, so more research is needed to determine the relationship between Dupixent and this type of cancer. Dupixent may alter the immune system in a way that opens the door for cutaneous T-cell lymphoma to develop. Similarly, it may unmask the condition in someone who has or is predisposed to have it. Nonetheless, given the strong association between Dupixent and cutaneous T-cell lymphoma, the study concludes that caution is warranted in diagnosing and treating eczema patients with Dupixent.
Who Can File a Dupixent Lawsuit?
People who want to file a lawsuit against the manufacturers of Dupixent need to meet certain eligibility criteria to open a case. Our attorneys are reviewing cases from people who were prescribed Dupixent and then developed one of the following conditions:
- Cutaneous T-Cell Lymphoma (CTCL)
- Mycosis fungoides
- Sézary Syndrome
- Lymphomatoid papulosis
- Anaplastic large cell lymphoma
- Extranodal NK-cell/T-cell lymphoma
- Subcutaneous panniculitis-like T-cell lymphoma
- Adult T-cell leukemia/lymphoma
- Follicular helper T-cell lymphoma
- Peripheral T-cell lymphoma
Additionally, to qualify for lawsuit, the patient must have took Dupixent after 2017 and been on the drug for at least 3-6 months. If you are not sure if you or a family member qualifies for a lawsuit, please reach to our firm for a case evaluation.
What Damages Can I Recover in a Dupixent Lawsuit?
Patients who take Dupixent and develop cutaneous T-cell lymphoma may be able to recover compensation for their associated losses. Damages they may be eligible for include:
- Medical costs
- Lost income and earning potential
- Pain and suffering
- Loss of quality of and enjoyment in life
- Wrongful death damages (recoverable by eligible family members if the patient dies because of cutaneous T-cell lymphoma)
Patients can support their claim for damages by presenting medical testing results (like biopsies), treatment records, prescription costs, and work history (including requests for time off for cancer treatments). Surviving family members may also be able to recover funeral and burial expenses, loss of financial and emotional support, mental anguish, and other forms of compensation.
Is There a Deadline to File a Dupixent Lawsuit?
Yes, there is a deadline to file a Dupixent lawsuit. Depending on the state you file your lawsuit in, the deadline is typically 1 to 2 years from the time you were diagnosed with cutaneous T-cell lymphoma or discovered that Dupixent may have caused your CTCL. This deadline is called the statute of limitations, and it varies by state and claim type. Typically, the deadline falls within a few years after the person discovers or could have discovered the connection between Dupixent and their cutaneous T-cell lymphoma diagnosis.
However, there are exceptions that can push this deadline forward or backward, and different deadlines apply to wrongful death claims. Swift action is recommended to minimize the risk of missing out on compensation due to missed due dates. Personal injury attorneys can help you determine what your deadline is and help you meet it.
What Are the Estimated Settlement Amounts in a Dupixent Lawsuit?
Dupixent settlement amounts will vary based on the person’s individual circumstances, the applicable state law, and other factors. Anticipated Dupixent settlements could range from $100,000 to $500,000. In general, people with severe or advanced cases of cutaneous T-cell lymphoma may receive a higher settlement. People who lost a loved one to cutaneous T-cell lymphoma may also be eligible for a higher payout. However, all settlements are decided on a case-by-case basis, so individual results may vary.
King Law Is Investigating Dupixent and T-Cell Lymphoma Claims
King Law and our legal partners are actively accepting and reviewing claims by patients who took Dupixent and developed cutaneous T-cell lymphoma. We have extensive experience fighting for consumers who were harmed by dangerous pharmaceutical products they were told would help them. Some of the benefits of working with us include:
- We do not charge upfront legal fees to hire us or book an appointment with us (we take all cases on contingency, meaning you only pay us if and when you win).
- We provide free case evaluations.
- We have a proven track record of helping patients who were hurt by dangerous drugs and medical devices.
- We have a robust network of resources to help us build strong cases.
- We have a dedicated, in-house intake team. You will not be sent to a generic call center.
- We are dedicated to helping people harmed by corporate negligence.
We treat our clients like people, not dollar figures. We take the time to get to know you, your concerns, and what a successful outcome means to you. Our full-service law firm is here to help you pursue justice because of how Dupixent has harmed you.
Contact a Dupixent Lawyer Today
If you took Dupixent and were diagnosed with cutaneous T-cell lymphoma, you may be eligible for a settlement. Contact us today by calling (585) 496-2648 to schedule a free, no-obligation consultation with an experienced attorney who can help. We can work with you to develop an effective case strategy to maximize your chances of winning a fair payout for your losses.

