People are filing spinal cord stimulator (SCS) lawsuits alleging the devices were defective and caused people to experience burns, infections, and repeat surgeries to repair or remove these devices. To date, the FDA has received more than 100,000 complaints about spinal cord stimulators. Companies like Boston Scientific, Medtronic, Abbott, and Nevro Corp are accused of not properly disclosing the dangerous side effects of these devices. Many scientific studies have shown spinal cord stimulators are prone to device migration and electric malfunction, which can lead to neurological injuries requiring additional treatment.
Individuals who have experienced complications after having the spinal cord stimulator implanted say they were not properly warned about these dangerous side effects by the manufacturers. After experiencing burns, revision surgery, and removal of the implant, many people suffered financial, physical, and mental setbacks. In an effort to gain compensation for these losses, people are filing spinal cord stimulator lawsuits.
Spinal Cord Stimulator Lawsuit – 2025 Updates
September 17, 2025: Study Shows Many Spinal Cord Stimulators Fail Within Three Years
Researchers at the University of Sydney have found that 23% of spinal cord stimulator patients “will require invasive surgical reintervention to correct issues with the stimulator hardware.” The researchers’ findings were published in the Medical Journal of Australia. also showed that most of those hardware failures occurred within three years after the device was implanted. The authors of the study stressed that these devices are often costly and ineffective, despite their reputation as being a preferred treatment for lower back pain. Patients who have suffered complications, including electrocution and burns, are suing the manufacturers to hold them accountable for not designing a safer device or telling the public about the risks.
September 4, 2025: Spinal Cord Stimulators Are Among Devices With Highest FDA Flag Rate
According to an analysis in the journal Interventional Pain Medicine, spinal cord stimulators (SCS) are one of the most commonly flagged medical devices on the market. According to data from FDA databases, SCS devices have caused more than 80,000 injuries in patients. This number of injury reports is high compared to other medical devices. The same paper examined information from legal databases and found that the average settlement for people hurt by spinal cord stimulators (SCS) was $1.9 million. These devices have caused life-altering injuries for thousands of Americans.
July 29, 2025: People Continue Filing Lawsuits Against Companies Who Make Faulty Spinal Cord Stimulator (SCS) Devices
More people are filing civil lawsuits against SCS manufacturers after they experienced debilitating injuries and additional surgeries to address their device’s malfunction. These lawsuits allege device manufacturers sidestepped FDA regulations and put unapproved medical devices in patients who were battling chronic pain.
May 2, 2025: Man in Minnesota Takes Aim at Medtronic for Defective Spinal Cord Stimulator
Dilman (Dilly) Anderson’s doctor implanted Medtronic’s Intellis spinal cord stimulator system in 2018 to help address his ongoing pain issues. A Medtronic service representative assisted with the procedure and helped Dilly in the hospital, and they assured Dilly then and afterwards that the device would deliver promised results. Dilly experienced bothersome and painful complications after having the device implanted, including painful electrical shocks, worsening nerve pain in his back and limbs, and the inability to control his bladder. Dilly says his doctor and the Medtronic representatives downplayed Dilly’s concerns about ongoing pain and new and worsening symptoms. After learning about the regulatory issues involved in this defective device, he filed against Medtronic for its faulty spinal cord stimulator.
April 29, 2025: Michigan Man Calls Out Boston Scientific in Spinal Cord Stimulator Lawsuit
Mark Dunham had the WaveWriter Alpha Spinal Cord Stimulator System implanted in March of 2023. Due to serious and bothersome complications, Mark had his doctor remove the device just 10 months later, in January of 2024. His doctor replaced it with a different Boston Scientific spinal cord stimulator model. Mark experienced startling and painful electrical jolts and nerve pain in the updated model. As instructed, Mark attempted to contact the Boston Scientific representative for help reprogramming the device. After a considerable delay, Mark received help from Boston Scientific, but the reprogramming efforts were unsuccessful, and the pain and electrical shocks continued. Mark learned of the numerous reports of electrical shocks, burns, and nerve pain experienced by others who had received spinal cord stimulator implants, which were not disclosed to him. He filed a lawsuit against Boston Scientific for his injuries related to his spinal cord stimulator device.
April 18, 2025: Medtronic Under Fire By Minnesota Woman for Its Restore or PrimeAdvanced Spinal Cord Stimulator
Angela Yates suffered from chronic, uncontrolled pain. After years of efforts to manage her pain, her doctor recommended that she have Medtronic’s spinal cord stimulator implanted. She was told this device was safe and effective and would help reduce her pain levels. However, the device overheated, burned her back, sent painful and jarring electrical jolts throughout her body, and worsened her pain symptoms. She discovered that Medtronic had modified the spinal cord stimulator’s design over a hundred times since getting FDA approval in 1984; however, the company had not gone through the approval process or clinical trials ever since. She has filed a lawsuit related to the SCS made by Medtronic and is demanding answers and justice.
March 19, 2025: Illinois Woman Files Lawsuit Against Proclaim Spinal Cord Stimulator System Maker, Abbott
Karen Krantz is suing Abbott because of the permanent harm caused by its defective Proclaim Neurostimulation spinal cord stimulator system. She accuses Abbott of not being transparent with the FDA and the public about the safety issues and risks with its Proclaim Neurostimulation System. Karen experienced debilitating nerve pain, shearing cold sensations, and painful electrical jolts caused by the Proclaim system. She is suing Abbott to recover compensation and hold the company accountable for the dishonest marketing and sales practices.
April 12, 2024: Man in New Mexico Sues Nevro Over Defective HF10 Spinal Cord Stimulator
Ron Yusnukis had a Nevro HF10 spinal cord stimulator implanted in March of 2021 to help treat his chronic back pain. During the trial period, the external device had relieved his pain; however, he started experiencing severe complications after having the full internal device implanted. He believes the device caused him to suffer a range of side effects, including losing control of his bowels, struggling with mobility and muscle spasms, and dangerous heart problems. Ron’s doctor removed the device; however, Ron still experiences permanent challenges because of the damage the device caused to his spinal cord. He is suing Nevro to recover damages for his permanent losses caused by Nevro’s SCS.
About the Spinal Cord Stimulator Lawsuit:
Legal Grounds in the Spinal Cord Stimulator Lawsuit
What Is Spinal Cord Stimulation?
How Does a Spinal Cord Stimulator Work?
Spinal Cord Stimulator Implant Symptoms and Side Effects
What Can Go Wrong With Spinal Cord Stimulators?
Severe Pain Reported After Spinal Cord Stimulator Surgery
Spinal Cord Stimulator Companies and Manufacturers Named in the Lawsuit
Who Qualifies to File a Spinal Cord Stimulator Lawsuit?
Recoverable Damages in the Spinal Cord Stimulator Lawsuit
What Proof Do I Need to File a Spinal Cord Stimulator Lawsuit?
How to File a Spinal Cord Stimulator Lawsuit
What Is the Deadline to File a Spinal Cord Stimulator Lawsuit?
Spinal Cord Stimulator Verdicts and Settlements
Legal Grounds in the Spinal Cord Stimulator Lawsuit
Patients are suing spinal cord stimulator manufacturers, such as Medtronic and Abbott, because these companies’ devices were defective and harmed patients. Patients say they were promised pain relief from spinal cord stimulator implants but, instead, they suffered severe injuries. Additionally, lawsuits filed by patients allege that the manufacturers modified the devices in ways that did not align with the FDA-approved designs, essentially sidestepping the need for a new FDA approval. Patients also believe their doctors were negligent in how they implanted and managed the spinal cord stimulators.
What Is Spinal Cord Stimulation?
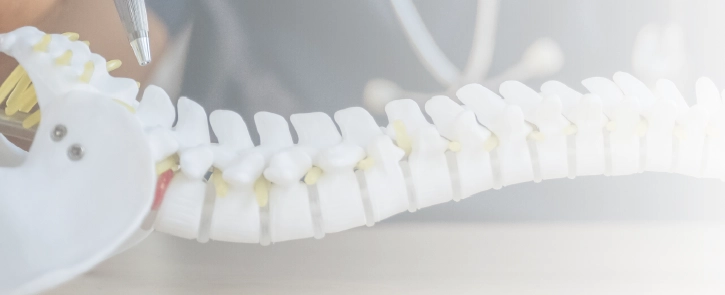
Spinal cord stimulation (also called neuromodulation therapy) is when a medical device sends electrical signals to nerves in the spine, with the goal of decreasing someone’s pain. To stimulate the spine, doctors implant a device called a spinal cord stimulator. These devices are sometimes known as pain pacemakers.
The spinal stimulation device has leads (like electrode stickers doctors put on your chest for an EKG), and these send electrical signals through the spinal cord. The device is implanted under the skin and often controlled and monitored wirelessly. This allows the patient and their medical team to see how the device is working and adjust the settings as needed.
Spinal cord stimulators are meant to be used after other surgical or medication-based treatments have been unsuccessful. Some of the conditions that spinal cord stimulators might be used to treat include nerve damage caused by diabetes (diabetic neuropathy), pain that does not go away after a back surgery (failed back surgery syndrome), and complex regional pain syndrome.
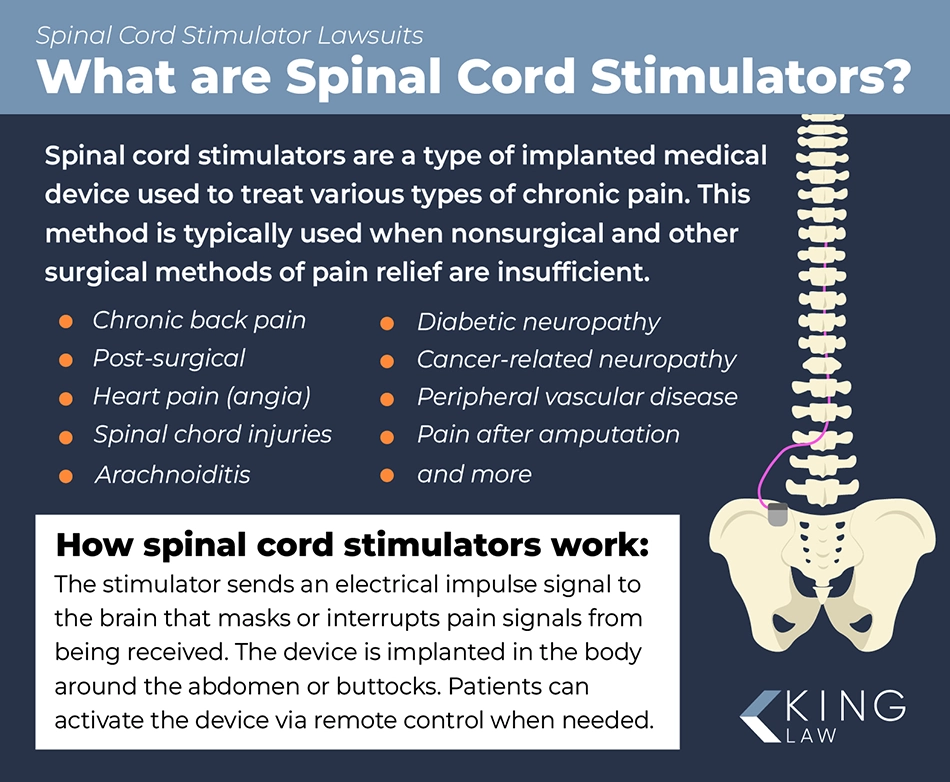
How Does a Spinal Cord Stimulator Work?
A spinal cord stimulator is a device that is implanted in someone’s back to help relieve severe or chronic pain (e.g., pain that does not go away). The device delivers a signal that helps to stop or mask the nerve pain signal, so the patient should feel a tingling or neutral sensation instead of shooting, sharp, or dull pain. The idea is that the spine can only send one signal to the brain at a time. So, the device sends a different sensation, so the brain does not receive the severe pain signal.
There are several different types of spinal cord stimulators. Some of them have rechargeable batteries that are implanted inside the patient. Others require the patient to have another surgery when their spinal cord stimulator’s battery runs out. Typically, patients can send the electrical signal themselves, which gives them more control over their pain relief. Spinal cord stimulator devices are controversial, and some medical professionals question whether they are truly effective, safe, and preferred over other, less-invasive measures.
Spinal Cord Stimulator Implant Symptoms and Side Effects
As with any procedure or medical implant, there is a range of possible side effects associated with spinal stimulators. Side effects of spinal cord stimulators include:
- Pain, discomfort, or mild swelling where the doctors implanted the device
- Tingling, twitching, or jolts of electricity
- Painful tingling
- Numbness
- Weakness
- Difficulty controlling or coordinating muscle movements
- Headaches
- Leaking spinal fluid
- Painful swelling or oozing from the implant site
- Sharp pain randomly or when moving
- Burning sensation
- Pain levels stay the same or worsen after the device is implanted
- Fever or chills
Some of these symptoms may show up right after you have the surgery to implant the spinal cord stimulator. However, these may appear days, weeks, or months later. You should report any signs to your doctor, because what you are experiencing may be the first stages of a serious adverse event. You may also be entitled to compensation if a defect in your spinal cord stimulator device harmed you.
FDA Recalls of Spinal Cord Stimulators
There has been a recall of Abbott spinal cord stimulators. The recall was issued after several Infinity and Proclaim stimulators would not turn back on after being placed in MRI mode. The recall was issued September 13, 2023. Many people whose Abbott stimulators failed after being placed in MRI mode had to have a revision surgery to have a new device implanted.
What Can Go Wrong With Spinal Cord Stimulators?
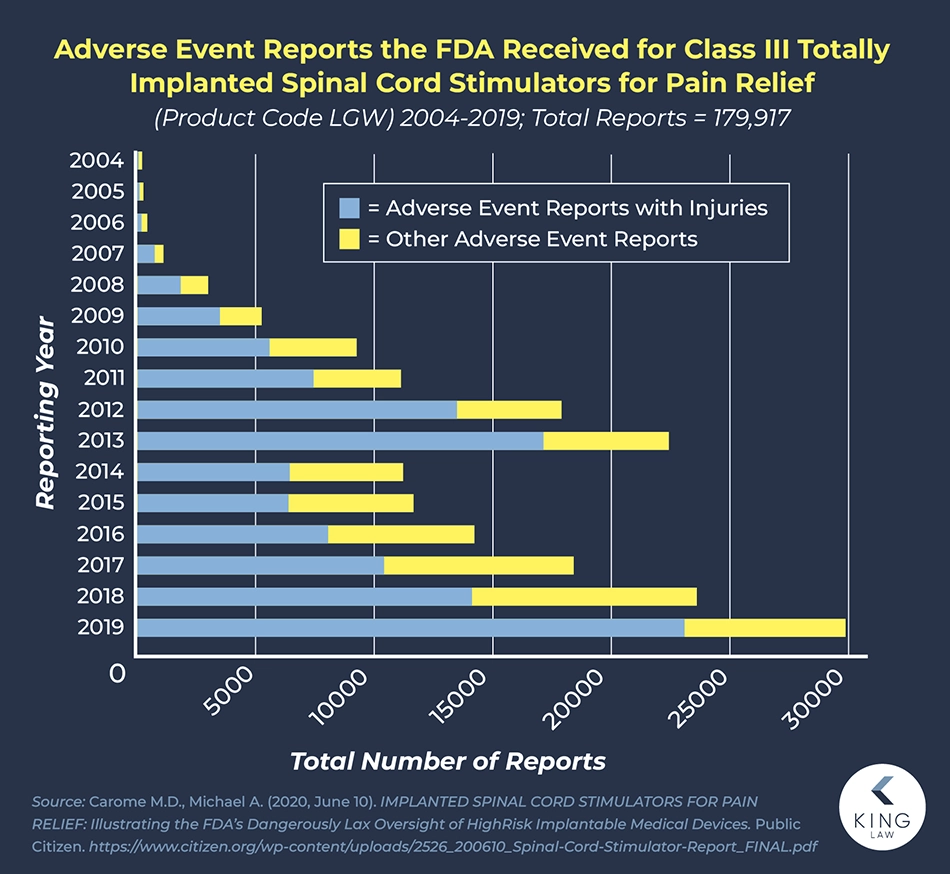
According to complaints filed with the FDA, spinal cord stimulators can experience a range of malfunctions that can harm patients. According to data compiled by Public Citizen, who searched the FDA’s MAUDE database, there were 179,917 reports filed against implanted spinal cord stimulators between 2004 and 2019. Of those incident reports, 118,272 involved injuries, and 23,311 resulted in revision surgeries.
Things that can go wrong with a spinal cord stimulator include:
- Lead migration: The leads that were initially implanted in the spine may migrate from their original placement.
- Lead fractures: The wires that carry the electrical pulses may fracture/break, meaning the desired therapy is not delivered.
- Device damage: The device may become damaged and not work properly, send unnecessary shocks, or stop working altogether.
- Device power failures: The device’s power supply may fail, meaning the patient does not get any therapeutic benefit or decrease in their pain. A malfunctioning battery may also cause burns.
When these malfunctions happen, the patient can experience:
- Infection
- Increased pain
- Electric shocks
- Burns
- New nerve pain
According to data from 2016 to 2020, the FDA received 497 reports associated with patients’ deaths, 77,937 with patients’ injuries, and 29,294 with device malfunction. Often, these side effects result in people needing revision surgeries and new devices implanted.
According to research, patients who experience severe complications should work with their doctors to address concerns immediately. Prompt treatment of neurological injuries, infections, and biological complications can improve a patient’s long-term outcome.
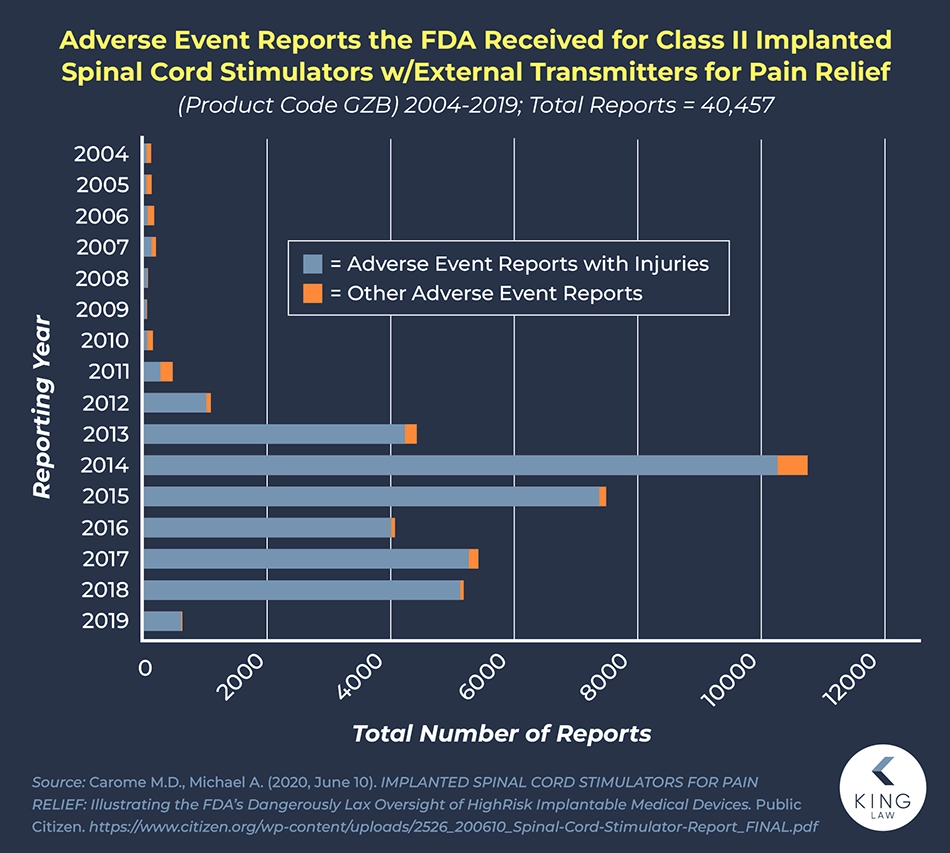
Severe Pain Reported After Spinal Cord Stimulator Surgery
Spinal cord stimulators promise to relieve patients’ chronic pain; however, many patients have experienced new or worsening symptoms after having this device implanted. According to one study, about 20 to 40 percent of patients experience complications after having a spinal cord stimulator implanted.
For example, there have been several reports of people experiencing a dangerous and painful blood clot after the implantation surgery. Others have suffered from excruciating pain, far beyond the pain the patient experienced before the spinal implant. Some of the devices did not work at all, or they could not be switched into or out of MRI mode, which disables the electrical signals to control pain.
Many patients have had to have the device removed, switched out, or revised because of the severe and troublesome complications they were experiencing. Additionally, several models of spinal cord stimulators have been recalled or have had warnings issued about them.
Spinal Cord Stimulator Companies and Manufacturers Named in the Lawsuit
Many well-known medical device companies are being called out in spinal cord stimulator lawsuits filed by patients and surviving loved ones. These include Abbott (St. Jude), Boston Scientific, Medtronic, and Nevro. The claims being filed against these pharmaceutical companies include failure to warn of known risks, violating regulations, and designing defective and dangerous products.
Boston Scientific
Boston Scientific is being sued because of issues with several of its spinal cord stimulator models, including WaveWriter and Precision models. These devices experienced a range of problems, including where a routine system check would trigger a device reset that would make it stop sending the pain-relieving signals.
Boston Scientific is also under fire for improper dealings with the FDA. Complaints allege that the company received FDA approval for a spinal cord stimulator design that was defective and later altered. Plaintiffs also believe Boston Scientific was dishonest with patients and providers about the true risks, like the likelihood of the device migrating or burning the patient.
Medtronic
Medtronic’s spinal cord stimulators have also received criticism, including its now-recalled Intellis, Inceptiv, Vanta, and Restore series of implants and accessories. For example, according to a complaint, Medtronic has changed the design and features of its spinal cord stimulator dozens of times since it received FDA approval in 1984. However, Medtronic has not notified the FDA or asked it to approve the updates. The complaint alleges that Medtronic has been marketing an unapproved version of the device for decades, putting hundreds of patients at risk. Medtronic has also been forced to pay millions in settlements because it paid physicians to get them to use Medtronic’s devices.
Abbott (formerly St. Jude Medical)
Abbott (which acquired St. Jude Medical in January 2017) has had many of its spinal cord stimulators recalled by the FDA. For example, in 2023, the FDA recalled the Proclaim and Infinity series of spinal cord stimulators because these models would not leave MRI mode. Abbott’s Eon Mini charging systems were also defective and recalled because they would not charge correctly or they would overheat and burn patients. These defective devices caused patients to suffer considerable pain, discomfort, and injuries, and ongoing lawsuits call on Abbott to answer for these and other harms their products inflicted.
Nevro Corp.
Nevro Corp. has faced several legal actions, including allegations of theft of trade secrets from competitor Boston Scientific and product liability actions filed by consumers. Many patients have suffered complications from Nevro’s high-frequency HF10 device. The electrodes (leads) in the device broke off or migrated, which caused severe pain and injuries to some patients. Battery problems also plague this model, with multiple reports of the battery glitching or not working at all.
Other Spinal Cord Stimulator Manufacturers
Other spinal cord stimulator manufacturers are being sued because their devices are causing pain and injury to patients. These include Saluda Medical, which makes the Evoke Closed Loop Stimulator system that some patients have had to have removed or revised after it was implanted. As more companies enter the spinal cord stimulator scene, they may be held accountable if they create defective devices or do not warn their patients about the range of risks to expect.
Who Qualifies to File a Spinal Cord Stimulator Lawsuit?
Patients who were harmed by defective spinal cord stimulators may be entitled to compensation. To be eligible for a settlement, the person needs to have a qualifying injury and have had a defective stimulator device implanted. Here are some of the injuries that people are suing spinal cord stimulator manufacturers about:
- Burns caused by the device or battery malfunction
- Electrocutions or electric shock caused by the device short-circuiting
- Lead wires (electrodes) migrating or breaking off
- Infections caused by the device after healing from the initial surgery
The manufacturers that are known to have made defective spinal cord stimulators include:
- Abbott (St. Jude’s)
- Medtronic
- Boston Scientific
- Nevro
The patient can file a direct, individual lawsuit against the pharmaceutical company that harmed them. The patient’s surviving loved ones may file a wrongful death claim if the patient dies due to complications from the defective device.
Recoverable Damages in the Spinal Cord Stimulator Lawsuit
Patients who experienced complications from a defective spinal cord stimulator may qualify for a range of compensation to address their losses. Payouts from a spinal cord stimulator lawsuit could include a financial award to cover the money they paid or lost because of their injuries. Additionally, they may be eligible for a settlement to address non-monetary damages, such as loss of quality of life.
Economic Damages
Complications from spinal cord stimulators can come with an enormous cost, including:
- The medical costs to repair, switch out, or reposition the device
- Hospitalizations and doctor visits to diagnose and treat complications
- Lost wages
- A reduction in earning capacity because of device complications
- The costs of future treatments, surgeries, or therapies related to the spinal cord stimulator defect
The victim’s compensation package could include all past, current, and future financial impacts of the injuries from the defective device.
Non-Economic Damages
The victim may also qualify for the social, emotional, physical, relational, and other costs associated with the spinal cord stimulator’s defects. These could include the following:
- Pain and suffering from going through the trauma caused by the defect
- Emotional distress
- The loss of quality of life or enjoyment in life because of the defect
- Permanent disfigurement or a permanent disability
The total amount of someone’s non-economic compensation package depends on factors like the extent of their losses and the proof they have to show they were harmed by the defective spinal cord stimulator.
What Proof Do I Need to File a Spinal Cord Stimulator Lawsuit?
To have a chance of winning your lawsuit, you need to be able to prove that you received a defective spinal cord stimulator and that it harmed you. Some of the evidence you might need includes the serial number for the device you received, medical records showing the date and location of your surgery, and the doctor’s notes about your complications. You might also save information about how your pain impacted your life, such as requests for time off work, a pain diary, or testimony from people who know you and your condition well. King Law can determine what evidence might be helpful in your situation and submit those requests to build a strong claim on your behalf.
How to File a Spinal Cord Stimulator Lawsuit
Filing a spinal cord stimulator claim requires you to follow a specific legal process. Some of the steps you might have to follow include:
- Step 1: Talking to a seasoned product liability or medical malpractice lawyer
- Step 2: Helping the attorney gather information about the spinal cord stimulator and how it impacted you
- Step 3: The lawyer writes up a legal complaint against the spinal cord stimulator’s manufacturer
- Step 4: The attorney prepares and responds to settlement proposals
- Step 5: Your attorney fights to get you a fair compensation package through the court system (if the at-fault pharmaceutical company does not settle)
King Law is prepared to help victims navigate the legal process of suing the companies that made defective spinal cord stimulators. We understand how much it can impact someone’s life when these devices malfunction and harm the person they were supposed to help.
What Is the Deadline to File a Spinal Cord Stimulator Lawsuit?
You need to file a spinal cord stimulator lawsuit within a specific amount of time. This timeframe is called the statute of limitations. The deadline is based on state law, and it can be 1 to 6 years from when you discover the defect in your spinal cord stimulator or from when you get hurt. However, in most states, the deadline is 1 to 2 years. There are exceptions that make this deadline longer or shorter, however. An attorney is a valuable resource who can help you determine what your filing deadlines are and how to meet those so you do not miss out on key compensation.
Spinal Cord Stimulator Verdicts and Settlements
The spinal cord stimulator lawsuits are still in their early stages in civil state court; however, some victims have received compensation. For example, a New Jersey man won a $1.25 million settlement in 2024 after his spinal cord stimulator compressed his spinal column and significantly worsened his pain and mobility challenges. Other victims may be awarded settlement packages that reflect the total losses they experienced because of the defective device.
Average Spinal Cord Stimulator Settlement Amount
There is no guaranteed or average amount that victims will receive; however, our attorneys estimate that some victims who have a successful claim may qualify for up to $250,000 to $650,000 if their injuries are severe. Factors that can impact the total settlement value include whether the person lost income or earning capacity because of the defective device and the medical treatments required to revise, remove, or switch out the spinal cord stimulator.
Contact a Spinal Cord Stimulator Lawyer Today
If you were harmed by a spinal cord stimulator, reach out to our law firm today by calling (585) 496-2648 or submitting an online contact form. We offer free consultations, and we will also review and evaluate your case for free. We have years of experience going up against giant pharmaceutical companies and holding them accountable for the harm their defective products inflict on unsuspecting patients.